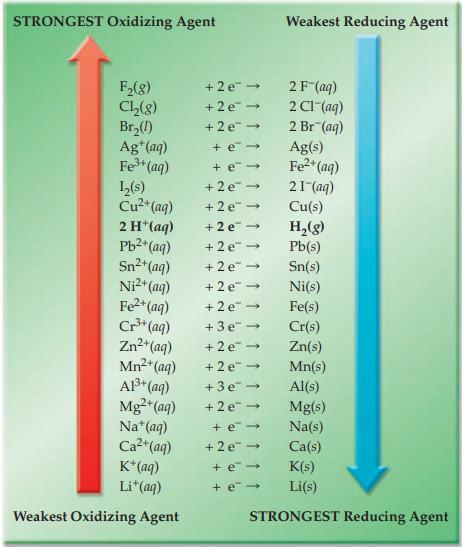
Refer to Figure 17.4 and indicate which substance in each of the following pairs is the stronger
Question:
Refer to Figure 17.4 and indicate which substance in each of the following pairs is the stronger oxidizing agent.
(a) F2(g) or Cl2(g)
(b) Ag+(aq) or Br2(l)
(c) Cu2+(aq) or H+(aq)
(d) Mg2+(aq) or Mn2+(aq)
Figure 17.4
Transcribed Image Text:
STRONGEST Oxidizing Agent F₂(8) Ch₂(8) Br₂(1) Ag+ (aq) Fe³+ (aq) L₂(s) Cu²+ (aq) 2 H+ (aq) Pb²+ (aq) Sn²+(aq) Ni²+ (aq) Fe²+ (aq) Cr³+ (aq) Zn²+(aq) Mn²+ (aq) A1³+ (aq) Mg²+ (aq) Na+ (aq) Ca²+ (aq) K+ (aq) Li+ (aq) Weakest Oxidizing Agent +2e™ → +2e → +2e → + e→ + e → +2e → +2e →> +2e → +2e → +2e → +2e → +2e →> +3e → +2e → +2e → +3e → +2e → Weakest Reducing Agent + e- +2e → + e- + e- 2 F (aq) 2 CI (aq) 2 Br(aq) Ag(s) Fe²+ (aq) 21- (aq) Cu(s) Sn(s) Ni(s) Fe(s) Cr(s) Zn(s) Mn(s) Al(s) Mg(s) Na(s) Ca(s) K(s) Li(s) STRONGEST Reducing Agent H₂(g) Pb(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
The answer correct is Mn2 is the stronger oxidizing agent compare...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 17.4 and indicate which substance in each of the following pairs is the stronger reducing agent. (a) Cu(s) or Cr(s) (b) H 2 (g) or Cu(s) (c) Cu(s) or I (aq) (d) Cl (aq) or H 2 (g) ...
-
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater tendency to be reduced. (a) Pb 2+ (aq) or Zn 2+ (aq) (b) Fe 3+ (aq) or Al 3+ (aq) (c) Ag + (aq) or I 2...
-
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater tendency to be oxidized. (a) Li(s) or K(s) (b) Al(s) or Mg(s) (c) Fe 2+ (aq) or I (aq) (d) Br (aq)...
-
In the diagram, the positive terminal of the 12 V battery is grounded it is at zero potential. At what potential is point X? 12 V 4 V Ground
-
Hidden Lakes Company purchased a delivery truck. The total cash payment was $30,020, including the following items. Negotiated purchase price ..... $24,000 Installation of special shelving .... 1,100...
-
Find the function value. Round to four decimal places. cot 146.15
-
Figure P27.38 shows a bar magnet placed at four positions on and near a spherical shell. Rank the positions according to the amount of magnetic flux through the shell, smallest flux first. Data from...
-
The following are the inventory for the years 2016, 2017, and 2018 for parry Company: Required: 1. Assume the inventory that existed at the end of each year was sold in the subsequent year. Prepare...
-
One key limitation of Sage 50 Accounting software, while there is a Sage 50 cloud version that offers some cloud-based features, the mobile access and functionality may not be as extensive as with...
-
Refer to Figure 17.4 and state whether each of the following reactions is spontaneous or nonspontaneous. (a) Br 2 (l) + LiF(aq) F 2 (s) + LiBr(aq) (b) Al(NO 3 ) 3 (aq) + Mn(s) Mn(NO 3 ) 2 (aq) +...
-
Chlorine can undergo a redox reaction in which it is simultaneously oxidized and reduced. Write a balanced equation for the following in a basic solution. Cl 2 (aq) ClO 2 (aq) + Cl (aq)
-
At the instant = 60, the construction lift is rotating about the z axis with an angular velocity of = 0.5 rad/s, and an angular acceleration of = 0.25 rad/s while the telescopic boom AB rotates...
-
What are the basic differences between the optimal block pulse function set and the non-optimal block pulse function set?
-
The Internet of things (IoT) connects you to the Internet in many ways. It could be your fitness tracker, your refrigerator, or your thermostat. Make a list of all the things you currently own that...
-
Create an FCB grid on an 11 8 piece of paper (that is, make the page horizontal). Now walk around your dorm room, apartment, or house choosing different things you see. Where would they fit on the...
-
Think about the cognitive dissonance model. Now think about a time when you bought something that, at the time, you didnt think it was a great deal but you later justified as a good deal. How did you...
-
Pick a brand that your parents might use but you dont. Look at ads for this brand and for its competition. Are they speaking to the same target audience? Or are these brands really reaching out to...
-
"I don't do any capital budgeting for buying my machines. They typically last for more than 10 years. If I have to do capital budgeting, I need to estimate my future cash inflows and outflows for the...
-
In Exercises 1-2, rewrite each verbal statement as an equation. Then decide whether the statement is true or false. Justify your answer. 1. The logarithm of the difference of two numbers is equal to...
-
On February 13, Elman Corporation issued for cash 75,000 shares of no-par common stock (with a stated value of $125) at $140. On September 9, Elman issued 15,000 shares of 1%, $60 preferred stock at...
-
The important dates in connection with a cash dividend of $112,750 on a corporations common stock are October 6, November 5, and December 5. Journalize the entries required on each date.
-
The important dates in connection with a cash dividend of $61,500 on a corporations common stock are July 1, August 1, and September 30. Journalize the entries required on each date.
-
Angela is a self-employed accountant who operates on a calendar year. On September 1st of this year, Anne paid $14,400 for lease of her office building. The rent covers the period Oct 1st of this...
-
Diego Company produces three styles of purses: style A, B, and C Style A Style B Style C Sales $210,000 $115,000 $60,000 Variable Expenses $64,000 $40,000 $40,000 Fixed Expenses $108,000 $60,000...
-
Problem. The flash point of a liquid mixture can be estimated by finding the temperature at which the equilibrium concentration of the flammable vapors in air reaches a concentration such that yi LFL...

Study smarter with the SolutionInn App


