Refer to Figure 17.4 and indicate which substance in each of the following pairs is the stronger
Question:
Refer to Figure 17.4 and indicate which substance in each of the following pairs is the stronger reducing agent.
(a) Cu(s) or Cr(s)
(b) H2(g) or Cu(s)
(c) Cu(s) or I–(aq)
(d) Cl–(aq) or H2(g)
Figure 17.4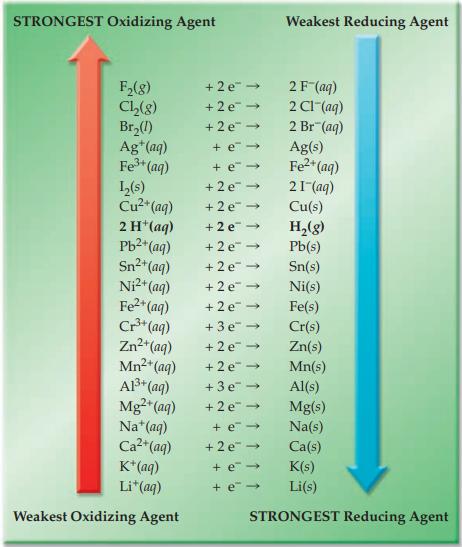
Transcribed Image Text:
STRONGEST Oxidizing Agent F₂(8) Ch₂(8) Br₂(1) Ag+ (aq) Fe³+ (aq) L₂(s) Cu²+ (aq) 2 H+ (aq) Pb²+ (aq) Sn²+ (aq) Ni²+ (aq) Fe²+ (aq) Cr³+ (aq) Zn²+ (aq) Mn²+ (aq) A1³+ (aq) Mg²+ (aq) Na+ (aq) Ca²+ (aq) K+ (aq) Li+ (aq) Weakest Oxidizing Agent +2e™ → +2e → +2e → + e→ + e → +2e → +2e → +2e → +2e → +2e → +2e → +2e → +3e → +2e → +2e → +3e → +2e → Weakest Reducing Agent + e- +2e → + e- + e- 2 F (aq) 2 CI (aq) 2 Br(aq) Ag(s) Fe²+ (aq) 21- (aq) Cu(s) Sn(s) Ni(s) Fe(s) Cr(s) Zn(s) Mn(s) Al(s) Mg(s) Na(s) Ca(s) K(s) Li(s) STRONGEST Reducing Agent H₂(g) Pb(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Crs...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 17.4 and indicate which substance in each of the following pairs is the stronger oxidizing agent. (a) F 2 (g) or Cl 2 (g) (b) Ag + (aq) or Br 2 (l) (c) Cu2 + (aq) or H + (aq) (d) Mg...
-
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater tendency to be reduced. (a) Pb 2+ (aq) or Zn 2+ (aq) (b) Fe 3+ (aq) or Al 3+ (aq) (c) Ag + (aq) or I 2...
-
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater tendency to be oxidized. (a) Li(s) or K(s) (b) Al(s) or Mg(s) (c) Fe 2+ (aq) or I (aq) (d) Br (aq)...
-
For the following exercises, find the inverse of the functions. f(x): || 3 x-4
-
DriveUp Taxi Service uses the units-of-activity method in computing depreciation on its taxicabs. Each cab is expected to be driven 150,000 miles. Taxi 10 cost $27,500 and is expected to have a...
-
Find the exact acute angle for the given function value. cot = 3
-
A \(1.00-\mathrm{m}\) metal bar that has a mass of \(0.900 \mathrm{~kg}\) is initially pinned in place on an incline \(65.0^{\circ}\) above the horizontal (Figure P27.37). There is a...
-
Hermosa, Inc. produces one model of mountain bike. Partial information for the company follows: Required: 1. Complete the table. 2. Calculate Hermosas contribution margin ratio and its total...
-
Other than the wind speed, what factor has the most impact on the amount of power generated by a wind turbine? Swept area of rotor Turbine style Terrain Blade material.
-
Refer to Figure 17.4 and state whether each of the following reactions is spontaneous or nonspontaneous. (a) Br 2 (l) + LiF(aq) F 2 (s) + LiBr(aq) (b) Al(NO 3 ) 3 (aq) + Mn(s) Mn(NO 3 ) 2 (aq) +...
-
Chlorine can undergo a redox reaction in which it is simultaneously oxidized and reduced. Write a balanced equation for the following in a basic solution. Cl 2 (aq) ClO 2 (aq) + Cl (aq)
-
Assume a cyclic machine that exchanges 6 kW with a 250oC reservoir and has a. Q.L = 0 kW, W. = 6 kW b. Q.L = 6 kW, W. = 0 kW and Q.L is exchanged with a 30oC ambient. What can you say about the...
-
Do you prefer Spotify, Pandora, Apple Music, or SoundCloud? What is the personality of each of these brands? Why do you prefer your choice to the others?
-
Walk around your room (or apartment or house), and pick a product that you use every day. What is the benefit of this product? That is, what does it do for you, the consumer? How can you sell that...
-
The Company is also making significant changes in manufacturing which will continue to increase margins through efficiency improvements, range changes and capital investment. Production activity is...
-
As we discussed in chapter 6, researchers should always start with secondary research before moving on to primary research, whether quantitative or qualitative. For this activity, approach your...
-
Develop a survey of three questions. For example, lets say you want to know where your friends would like to take a road trip, and you might ask them that open-ended question. Lets say you get five...
-
In Chapters 9 and 10, we used cost allocations to estimate the cost of capacity resources, and to measure the long-term profitability of investing in resources and products. In what ways does this...
-
Three forces with magnitudes of 70pounds, 40 pounds, and 60 pounds act on an object at angles of 30, 45, and 135, respectively, with the positive x-axis. Find the direction and magnitude of the...
-
Self Storage Corporation has 100,000 shares of $40 par common stock outstanding. On May 10, Self Storage Corporation declared a 2% stock dividend to be issued August 1 to stockholders of record on...
-
Spectrum Corporation has 600,000 shares of $75 par common stock outstanding. On February 13, Spectrum Corporation declared a 4% stock dividend to be issued April 30 to stockholders of record on March...
-
On October 3, Valley Clothing Inc. reacquired 10,000 shares of its common stock at $9 per share. On November 15, Valley Clothing sold 6,800 of the reacquired shares at $12 per share. On December 22,...
-
Bill Johnson's will provides that $10,000 a year would be paid to his widow and $5,000 a year to his son out of the estate's income. There were no charitable contributions made. If the estate's...
-
DEF Company declares and distributes a 30% common stock dividend when it has 60,000 shares of $10 par common stock outstanding. The market price per share is $75 at the date of declaration. Which...
-
How much will Haveford Company move out of finished goods for the month of March? Beginning balances on March 1st are below: Raw materials: $2,000 Work in process: $1,000 Finished goods: $9,000 In...

Study smarter with the SolutionInn App


