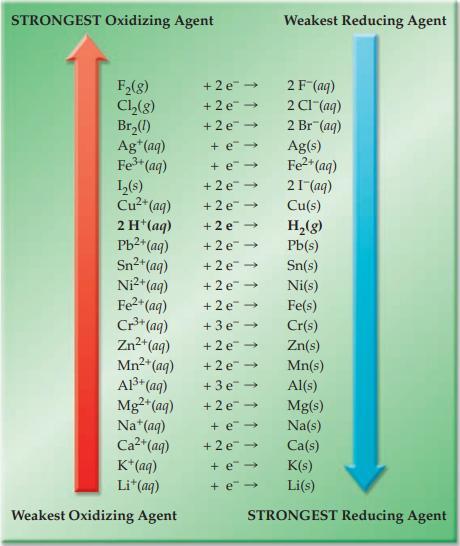
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater
Question:
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater tendency to be reduced.
(a) Pb2+(aq) or Zn2+(aq)
(b) Fe3+(aq) or Al3+(aq)
(c) Ag+(aq) or I2(s)
(d) Cu2+(aq) or Br2(l)
Figure 17.4
Transcribed Image Text:
STRONGEST Oxidizing Agent F₂(8) Cl₂(g) Br₂(1) Ag+ (aq) Fe³+ (aq) L₂(s) Cu²+(aq) 2 H+ (aq) Pb²+ (aq) Sn²+ (aq) Ni²+ (aq) Fe²+ (aq) Cr³+ (aq) Zn²+(aq) Mn²+ (aq) Al³+ (aq) Mg²+ (aq) Na*(aq) Ca²+ (aq) K+ (aq) Li+ (aq) Weakest Oxidizing Agent + 2 e + 2e → +2e →>> + e → + e-> +2e → +2e →>> +2e → +2e → +2e → +2e → +2e →> +3e → +2e →> +2e → +3e → +2e → Weakest Reducing Agent + e→ +2e → + e→ + e→ 2F-(aq) 2 C1-(aq) 2 Br (aq) Ag(s) Fe²+ (aq) 21 (aq) Cu(s) H₂(g) Pb(s) Sn(s) Ni(s) Fe(s) Cr(s) Zn(s) Mn(s) Al(s) Mg(s) Na(s) Ca(s) K(s) Li(s) STRONGEST Reducing Agent
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Pb2aq or Zn2aq The standard reduction potential E for Pb2 Pb i...View the full answer

Answered By

Ajeet Singh
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students. Areas of interest: Business, accounting, Project management, sociology, technology, computers, English, linguistics, media, philosophy, political science, statistics, data science, Excel, psychology, art, history, health education, gender studies, cultural studies, ethics, religion. I am also decent with math(s) & Programming. If you have a project you think I can take on, please feel welcome to invite me, and I'm going to check it out!
5.00+
4+ Reviews
24+ Question Solved
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:
Students also viewed these Sciences questions
-
Refer to Figure 17.4 and indicate which substance in each of the following pairs has the greater tendency to be oxidized. (a) Li(s) or K(s) (b) Al(s) or Mg(s) (c) Fe 2+ (aq) or I (aq) (d) Br (aq)...
-
Which substance in each of the following pairs would you expect to have the higher boiling point? Explain why. (a) Ne or Xe, (b) CO2 or CS2, (c) CH4 or Cl2, (d) F2 or LiF, (e) NH3 or PH3
-
According to general trends in the periodic table, predict which element in each of the following pairs has the greater metallic character. (a) B or Al (b) Na or K (c) Mg or Ba (d) H or Fe. Periodic...
-
Refer to E 29 and respond to the following requirements. Data in E 2-9 Prepare the necessary adjusting entries on December 31, 2024, for the Microchip Company for each of the following situations....
-
Depreciation information for Alan Chemicals Company is given in BE9-3. Assuming the declining-balance depreciation rate is double the straight-line rate, compute annual depreciation for the first and...
-
Find the function value. Round to four decimal places. cos ( -295.8)
-
An arbitrary-shaped tangle of wire is connected such that it carries a current \(I_{0}\) from position \(\vec{r}_{1}\) to position \(\vec{r}_{2}\) in a region where there is an external uniform...
-
Speier Company estimates that 240,000 direct labor hours will be worked during 2014 in the Assembly Department. On this basis, the following budgeted manufacturing overhead data are computed. It is...
-
Suppose we have a nMOSCAP made of Si. Suppose the channel doping is too strong at p = 1019 cm-3. All plots should assume the metal is on the left side. For this problem, we apply Vgs = Vth. (a) Plot...
-
Refer to Figure 17.4 and indicate which substance in each of the following pairs is the stronger reducing agent. (a) Cu(s) or Cr(s) (b) H 2 (g) or Cu(s) (c) Cu(s) or I (aq) (d) Cl (aq) or H 2 (g) ...
-
Chlorine can undergo a redox reaction in which it is simultaneously oxidized and reduced. Write a balanced equation for the following in a basic solution. Cl 2 (aq) ClO 2 (aq) + Cl (aq)
-
If the given sequence is arithmetic, find the common difference d. If the sequence is not arithmetic, say so. 2, -4, 6, -8, 10, -12, . . .
-
POEM is an acronym for paid, owned, and earned media. Give three examples of each of these in the media you use every day. Which do you think are the most effective? Why?
-
Comfy Chairs Co manufactures a standard office chair in Division A. The standard chair is improved in Division B with extra cushioning and easy-run castors. The manager of Division A has offered...
-
What are bankers looking for when they evaluate working capital.
-
Positioning can also become a brainstorming technique. Think of two opposites for two different points of interest for your target audience. Your X-axis might be a place and your Y-axis might be...
-
What is e-business.
-
Why do many firms begin projects on a small scale before making considerable investments?
-
Gordon and Lisa estimate that they will need $1,875,000 in 40 years for their retirement years. If they can earn 8 percent annually on their funds, how much do they need to save annually?
-
On February 1, Motorsports Inc. reacquired 7,500 shares of its common stock at $30 per share. On March 15, Motorsports sold 4,500 of the reacquired shares at $34 per share. On June 2, Motorsports...
-
Using the following accounts and balances, prepare the Stockholders Equity section of the balance sheet. Seventy thousand shares of common stock are authorized, and 7,500 shares have been reacquired....
-
Using the following accounts and balances, prepare the Stockholders Equity section of the balance sheet. Seventy thousand shares of common stock are authorized, and 7,500 shares have been reacquired....
-
Below is the direct materials budget for Carreras Company: Direct materials February $5,000 March $7,000 April $5,000 May $6,000 Of the purchases, 30% are cash and 70% are on account. Carreras...
-
If the variable cost per unit to produce a skateboard is $15 and the contribution rate is 30%, what is the contribution margin?
-
Explain why do we call retained earnings "earned capital"? A Company was founded in 2013. Its yearly earnings and dividend payments are own here: 2013: Net income of $4,000, paid zero dividends 2014:...

Study smarter with the SolutionInn App


