Question: Singly ionized helium He + is hydrogenlike. (a) Construct a carefully scaled energy-level diagram for He + similar to that in Figure 4-16, showing the
Singly ionized helium He+ is hydrogenlike.
(a) Construct a carefully scaled energy-level diagram for He+ similar to that in Figure 4-16, showing the levels for n = 1, 2, 3, 4, 5, and ∞.
(b) What is the ionization energy of He+?
(c) Compute the difference in wavelength between each of the first two lines of the Lyman series of hydrogen and the first two lines of the He+ Balmer series. Be sure to include the reduced mass correction for both atoms.
(d) Show that for every spectral line of hydrogen, He+ has a spectral line of very nearly the same wavelength. (Mass of He+= 6.65 x 10-27 kg.)
Figure 4-16
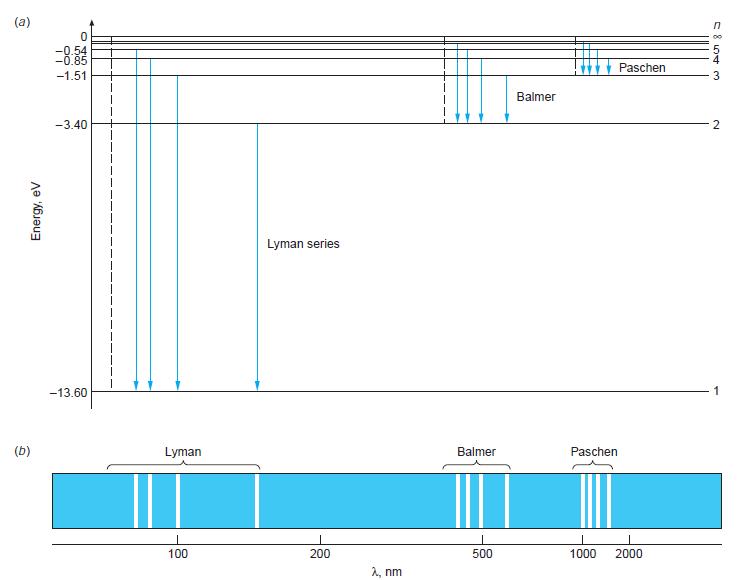
(a) Energy, eV (b) -0.54 -0.85 -1.51 -3.40 -13.60 I Lyman 100 Lyman series 200 , nm Balmer I 500 Balmer Paschen Paschen 1000 2000 S 5 844 3 2 1
Step by Step Solution
3.29 Rating (158 Votes )
There are 3 Steps involved in it
For He E n 136eVZ 2 n 2 544eVn 2 Equation 420 a b Ionization e... View full answer

Get step-by-step solutions from verified subject matter experts


