A vaporliquid mixture of ethylbenzene and toluene has a partial pressure of 250 mm Hg of ethylbenzene
Question:
A vapor–liquid mixture of ethylbenzene and toluene has a partial pressure of 250 mm Hg of ethylbenzene and 343 mm Hg of toluene. Write a MATLAB program that will compute the composition of the liquid phase and the temperature of this mixture if we assume ideal gas and liquid behavior. You can approximate the saturation pressures using the Antoine equation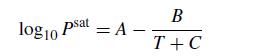
where the saturation pressure is inmmHg and the temperature is in Celsius. The Antoine coefficients are in the table below.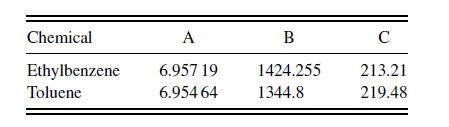
Your program should output the temperature and liquid-phase mole fractions of each species. What are the equations used for the residual and the Jacobian?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Numerical Methods With Chemical Engineering Applications
ISBN: 9781107135116
1st Edition
Authors: Kevin D. Dorfman, Prodromos Daoutidis
Question Posted:





