Equations 5.25ac on p. 193 show the formation of trialkylboranes from alkenes and BH 3 . In
Question:
Equations 5.25a–c on p. 193 show the formation of trialkylboranes from alkenes and BH3. In the reaction of 2,3-dimethyl-2-butene with BH3, only two equivalents of the alkene react, even with a large excess of alkene, to give a dialkylborane called disiamylborane.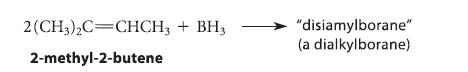
Give the structure of disiamylborane, and suggest a reason that only two equivalents of alkene react.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





