Predict the product formed when the conjugate-base enolate ion of ethyl 2-methylpro panoate (shown in Eq. 22.81)
Question:
Predict the product formed when the conjugate-base enolate ion of ethyl 2-methylpro panoate (shown in Eq. 22.81) is treated with bromobenzene and a catalytic amount of Pd[P(t-Bu)3]4, and explain the role of the catalyst.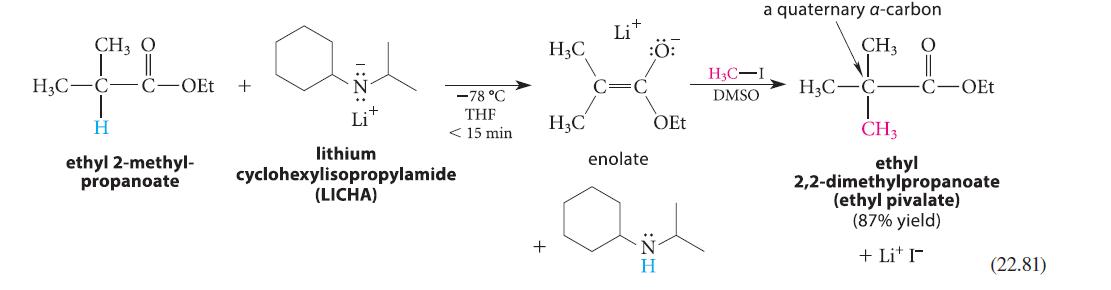
Transcribed Image Text:
CH3 O floo. Agh Lit H3C-C- C -OEt + H ethyl 2-methyl- propanoate -78 °C THF < 15 min lithium cyclohexylisopropylamide (LICHA) H3C H3C + Lit :O: enolate OEt H a quaternary a-carbon CH3 H3C-I DMSO H3C-C- CH3 OEt ethyl 2,2-dimethylpropanoate (ethyl pivalate) (87% yield) + Li+I (22.81)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
This is a transitionmetal catalyz...View the full answer

Answered By

Emily Grace
With over a decade of experience providing top-notch study assistance to students globally, I am dedicated to ensuring their academic success. My passion is to deliver original, high-quality assignments with fast turnaround times, always striving to exceed their expectations.
4.90+
3+ Reviews
22+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The reactions of ester enolate ions are not restricted to simple alkylations. With this in mind, suggest the structure of the product formed when the enolate ion formed by the reaction of fm-butyl...
-
The compound BD3 is a deuterated form of borane. Predict the product formed when 1-methylcyclohexene reacts with BD3 THF ,followed by basic hydrogen peroxide.
-
A major component of olive oil is glyceryl trioleate. (a) Give the structures of the products expected from the saponification of glyceryl trioleate with excess aqueous NaOH. (b) Give the structure...
-
Francis and Peter are in a partnership sharing profits and losses in the ratio 3:2. The following is their trial balance as at 30 September 2020. particulars D ebit C redit Buildings (cost: RM...
-
Refer back to the California Pool Supplies inventory data in Short Exercise 6-12. Requirement 1. How would the inventory error affect California Pool Supplies cost of goods sold and gross profit for...
-
A bank has issued a six- month, $ 2 million negotiable CD with a 0.52 percent quoted annual interest rate (i CD, sp ). a. Calculate the bond equivalent yield and the EAR on the CD. b. How much will...
-
List three ways to get input from the console and convert that input to the desired data type.
-
Accounting for Goodwill Fred Graf, owner of Graf Interiors, is negotiating for the purchase of Terrell Galleries. The balance sheet of Terrell is given in an abbreviated form below. Graf and Terrell...
-
4.4.4. The shareholders of Zedzee Pty Ltd are Sufyaan (10 shares), Shahedah (10 shares), Hilton (10 shares) and Sue (10 shares). The directors are Sufyaan and Shahedah. Upon registration, Zedzee...
-
Indicate whether each of the following compounds could be prepared by a malonic ester synthesis. If so, outline a preparation from diethyl malonate and any other reagents. If not, explain why. (a)...
-
Outline a synthesis of each of the following compounds from either diethyl malonate or ethyl acetate. Because the branched amide bases are relatively expensive, you may use them in only one reaction....
-
For an axisymmetrical body under no force, prove (a) That the rate of retrograde precession can never be less than twice the rate of spin of the body about its axis of symmetry, (b) That in Fig....
-
The vast majority of all companies are valued using the following three primary steps to determine the [1] Total Purchase Price and [2] Debt and Access Cash: 1) Determine the enterprise value by...
-
A line vortex of strength I m2/s is located at a distance of L m to the right of a flat wall. A uniform up-flow of U! m/s acts on the vortex so that the vortex is stationary. The effects of gravity...
-
Find f'(x) if f (x) = log (cot 8x).
-
What are the main advantage of choosing schoolchildren as a target market for your backpack business? Give 4 major points.
-
A car of mass 1289 kg accelerates from rest to 22.9 m/s in 5.90 s. How much force was required to do this?
-
List four examples of customer acceptance measures.
-
Describe a group you belong or have belonged discuss the stages of group development and suggest how to improve the group effectiveness by using the group development model.
-
Sketch the 1H NMR spectrum you would expect for the following compound, showing the splitting patterns and relative position of each signal. CI CI Cl CI CI CI
-
List the following radicals in order of decreasing stability:
-
Consider the monochlorination of 2-methylbutane. (a) Assuming that the product mixture was subjected to fractional distillation, which fractions, if any, would show optical activity? (b) Could any of...
-
How do customers determine how much they are willing to pay for a good or service? based on the value of distinctive competencies based on the degree of process innovation based on the value they get...
-
A resource is already assigned to work on another task for a percentage of their time. You want to assign the rest of their available time during the same period to a second task. What is the field...
-
Analysis of a current business situation or event In this section you will be applying the work you completed in the prior section to a current business situation ( current would be within the last 6...

Study smarter with the SolutionInn App


