Question: Below are mass spectra for four different compounds. Identify whether each of these compounds contains a bromine atom, a chlorine atom, or neither. a. b.
Below are mass spectra for four different compounds. Identify whether each of these compounds contains a bromine atom, a chlorine atom, or neither.
a.
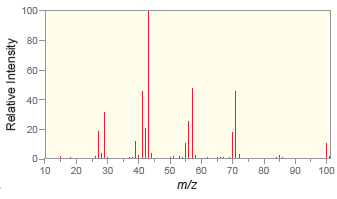
b.
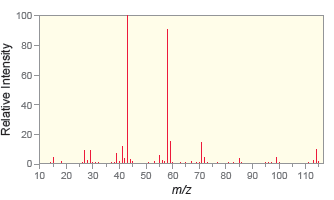
c.
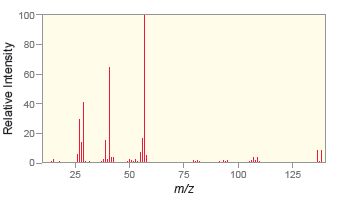
d.
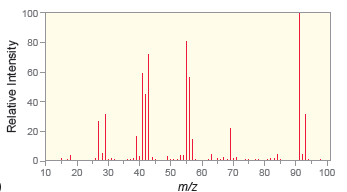
100- 80- 60- 60- 40 20- 0- 60 70 10 20 30 40 50 80 90 100 m/z Relative Intensity 100 80- 40 ot 10 20 30 40 50 60 70 80 06 100 110 m/z 80 20 Relative Intensity
Step by Step Solution
3.37 Rating (163 Votes )
There are 3 Steps involved in it
a There is not a significant M2 peak so neither bromine nor chlorine are present ... View full answer

Get step-by-step solutions from verified subject matter experts


