A piston-fitted cylinder with a 6-cm inner diameter contains 1.40 g of nitrogen. The mass of the
Question:
A piston-fitted cylinder with a 6-cm inner diameter contains 1.40 g of nitrogen. The mass of the piston is 4.50 kg, and a 25.00-kg weight rests on the piston. The gas temperature is 30°C, and the pressure outside the cylinder is 2.50 atm.
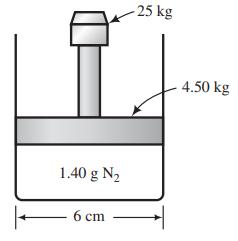
(a) Prove that the absolute pressure of the gas in the cylinder is 3.55x105 Pa. Then calculate the volume occupied by the gas, assuming ideal-gas behavior.
(b) Suppose the weight is abruptly lifted and the piston rises to a new equilibrium position. Further suppose that the process takes place in two steps: a rapid step in which a negligible amount of heat is exchanged with the surroundings, followed by a slow step in which the gas returns to 30°C. Considering the gas as the system, write the energy balances for step 1, step 2, and the overall process. In all cases, neglect ΔEk and ΔEp. If Û varies proportionally with T, does the gas temperature increase or decrease in step 1? Briefly explain your answer.
(c) The work done by the gas equals the restraining force (the weight of the piston plus the force due to atmospheric pressure) times the distance traveled by the piston. Calculate this quantity and use it to determine the heat transferred to or from (state which) the surroundings during the process.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-1119498759
4th edition
Authors: Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard





