Question: Deduce the relation between the pressure and mass density, , of a perfect gas of molar mass M. Confirm graphically, using the following data on
Deduce the relation between the pressure and mass density, ρ, of a perfect gas of molar mass M. Confirm graphically, using the following data on dimethyl ether at 25°C, that perfect behaviour is reached at low pressures and find the molar mass of the gas.
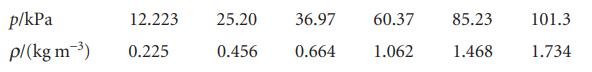
p/kPa p/(kg m-) 12.223 0.225 25.20 36.97 60.37 0.456 0.664 1.062 85.23 1.468 101.3 1.734
Step by Step Solution
3.41 Rating (167 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


