Question: Derive two additional mutually orthogonal hybrid orbitals for the lone pairs on oxygen in H 2 O, each of which is orthogonal to Ï a
Derive two additional mutually orthogonal hybrid orbitals for the lone pairs on oxygen in H2O, each of which is orthogonal to ψaand ψb, by following these steps:
a. Starting with the following formulas for the lone pair orbitals
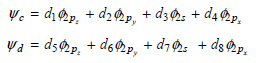
use symmetry conditions to determine d2 and d4, and to determine the ratio of d3 to d7 and of d4 to d8.
b. Use the condition that the sum of the squares of the coefficients over all the hybrid orbitals and lone pair orbital is 1 to determine the unknown coefficients.
dz, + d42p. V. = di2p, + d22p, + dz2; + d4 p. d22p, + dg p. - dg2p, ds2p. Va = dsp, + dsp, + d72:
Step by Step Solution
3.28 Rating (174 Votes )
There are 3 Steps involved in it
The lone pair orbitals have the form Can the calculation of the coefficients be simplified We do so ... View full answer

Get step-by-step solutions from verified subject matter experts


