Question: The reaction rate as a function of initial reactant pressures was investigated for the reaction 2NO(g) + 2H 2 (g) N 2 (g) + 2H
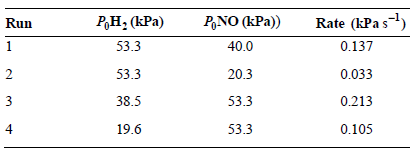
What is the rate law expression for this reaction?
Rate (kPa s) 0.137 P,NO (kPa)) ,: (Pa) Run 53.3 40.0 2 53.3 20.3 0.033 38.5 53.3 0.213 0.105 53.3 19.6 4
Step by Step Solution
3.42 Rating (165 Votes )
There are 3 Steps involved in it
The rate law expression is Rate kP H2 P NO We first find the order of the reaction U... View full answer

Get step-by-step solutions from verified subject matter experts


