a. Calculate the de Broglie wavelength of the electron in the n = 1, 2, and 3
Question:
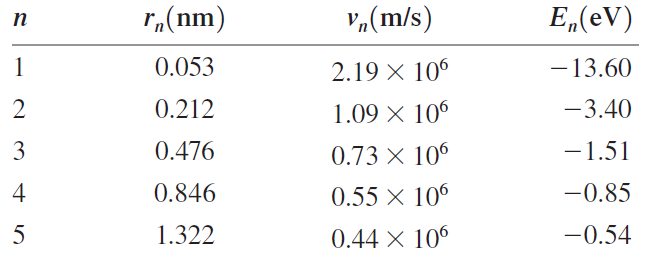 b. Show numerically that the circumference of the orbit for each of these stationary states is exactly equal to n de Broglie wavelengths.
b. Show numerically that the circumference of the orbit for each of these stationary states is exactly equal to n de Broglie wavelengths.
c. Sketch the de Broglie standing wave for the n = 3 orbit
Transcribed Image Text:
r„(nm) "¼(m/s) E„(eV) п 0.053 -13.60 2.19 X 106 2 0.212 -3.40 1.09 X 106 3 0.476 -1.51 0.73 × 106 4 0.846 0.55 × 106 -0.85 5 1.322 -0.54 0.44 X 10°
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
Solve a Using the data in Table 382 the wavelength of the electron in the n ...View the full answer

Answered By

Diane Joyce Pastorin
Please accept my enthusiastic application to solutioninn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group.
4.60+
2+ Reviews
10+ Question Solved
Related Book For 

Physics for Scientists and Engineers A Strategic Approach with Modern Physics
ISBN: 978-0133942651
4th edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Physics questions
-
A neutron in a reactor has kinetic energy of about 0.02 eV. Calculate the de Broglie wavelength of this neutron from Equation 17-12, where mc2 = 940 MeV is the rest energy of the neutron. hc 1240 eV...
-
We saw in Impact I8.1 that electron microscopes can obtain images with several hundredfold higher resolution than optical microscopes because of the short wavelength obtainable from a beam of...
-
Why Don't We Diffract? (a) Calculate the de Broglie wavelength of a typical person walking through a doorway. Make reasonable approximations for the necessary quantities. (b) Will the person in part...
-
Kim and Kanye have been dating for years and are now thinking about getting married. As a financially sophisticated couple, they want to think through the tax implications of their potential union....
-
Astronomers view light coming from distant galaxies moving away from Earth at speeds greater than 10% of the speed of light. How fast does this light meet the telescopes of the astronomers?
-
Most college students either currently have, or at one time have had, roommates or housemates. Think about a time when you have shared your living space with one or more students, and describe the...
-
To investigate the average time to failure of a certain soldered object subject to continuous flow of current, 5 soldered objects were subjected to specified volts and amperes of current and their...
-
You are the manager of a restaurant that delivers pizza to college dormitory rooms. You have just changed your delivery process in an effort to reduce the mean time between the order and completion...
-
If a sensor (I1:0) is used to count the incoming cars, and another sensor (I1:1) is used to count the outgoing cars, please design a PLC program such that when the number of cars in the parking lot...
-
ABCMobile is a mobile network operator headquartered in New Delhi, India. The company has enlisted your help as a consultant to develop and test a model on the determinants of subscriber churn in the...
-
a. What quantum number of the hydrogen atom comes closest to giving a 100-nm-diameter electron orbit? b. What are the electrons speed and energy in this state?
-
What is the radius of a hydrogen atom whose electron moves at 7.3 10 5 m/s?
-
Baubles, a jewelry store, contacts United Parcel Service (UPS) to ship a diamond ring worth $105,000. The owner of the store arranges for the shipment on UPSs website, which requires the customer to...
-
Question 6: A quantum particle of mass m is inside a one dimensional box of length L. Suppose that L is so small is not valid anymore 2m that the energy of the quantum particle has to be treated...
-
Write funny closing remarks for a wedding reception.
-
Jammer Corporation holds cash of $8,000 and owes $28,000 on accounts payable. Jammer has accounts receivable of $41,000, inventory of $24,000, and land that cost $60,000. How much are Jammer's total...
-
How do fluctuations in the global economy impact the valuation of bonds and stocks, and how can investors navigate these dynamics to make informed investment decisions? Provide an example to support...
-
Stock and bond valuation may differ based on geographical location. Identify 3 factors that create these differences. Choose two countries to use as an example and explain how these factors impact...
-
An 800-W iron is left on the iron board with its base exposed to the air at 20°C. The convection heat transfer coefficient between the base surface and the surrounding air is 35 W/m 2 ·K....
-
Use critical values to test the null hypothesis H0: 1 2 = 20 versus the alternative hypothesis H0: 1 2 20 by setting a equal to .10, .05, .01, and .001. How much evidence is there that the...
-
Pipes often contain solid residue that adheres to the inner wall of the pipe. If these pipes are not properly cleaned before use, then the flow of water through the pipe will slowly dissolve the...
-
Natural gas from a hydraulic fracturing process contains hydrogen sulfide (H 2 S) gas that will be removed by absorption into a specialty solvent through an interphase mass transfer process. In the...
-
A 15-cm-OD pipe is buried with its centerline 1.25 m below the surface of the ground (k of soil is 0.35 W/(m K)). An oil having a density of 800 kg/m 3 and a specific heat of 2.1 kJ/(kg K) flows in...
-
10. Create the following row vector A where it has 18 elements (1 to 18). 1 4 7 10 13 16. 11 14 17 a. Use the reshape function to obtain B: == 2 5 8 3 6 9 12 15 18 b. Create a 7 element row vector...
-
7) Use Matlab codes to create two different vectors to separately store the numerators and denominators of the following sequence of 50 fractional numbers: 1/3, 2/5, 3/7, 4/9, 5/11, 6/13, ......,...
-
US exports to China have been rapidly increasing but not fast enough to offset the imports from China. This means that the United States has a/an? Give answer and explain.

Study smarter with the SolutionInn App


