Question: A molecular bond can be modeled as a spring between two atoms that vibrate with simple harmonic motion. FIGURE P15.63 shows an SHM approximation for
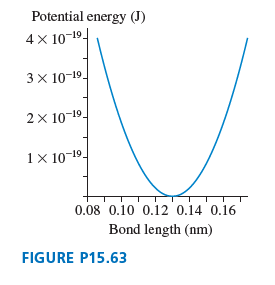
Potential energy (J) 4 x 10-19- 3x 10-19- 2x 10-19- 1 10-19- 0.08 0.10 0.12 0.14 0.16 Bond length (nm) FIGURE P15.63
Step by Step Solution
3.44 Rating (176 Votes )
There are 3 Steps involved in it
Solve The potential energy curve of a simple harmonic oscillator is des... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
1442_6054778b88bb7_693155.pdf
180 KBs PDF File
1442_6054778b88bb7_693155.docx
120 KBs Word File


