Question: Two excited energy levels are separated by the very small energy difference E. As atoms in these levels undergo quantum jumps to the ground state,
Two excited energy levels are separated by the very small energy difference ΔE. As atoms in these levels undergo quantum jumps to the ground state, the photons they emit have nearly identical wavelengths λ.
a. Show that the wavelengths differ by
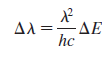
b. In the Lyman series of hydrogen, what is the wavelength difference between photons emitted in the n = 20 to n = 1 transition and photons emitted in the n = 21 to n = 1 transition?
Step by Step Solution
There are 3 Steps involved in it
Solve a To derive the formula consider the change in the energy of the p... View full answer

Get step-by-step solutions from verified subject matter experts


