Question: FIGURE P41.42 shows a few energy levels of the mercury atom. a. Make a table showing all the allowed transitions in the emission spectrum. For
FIGURE P41.42 shows a few energy levels of the mercury atom.
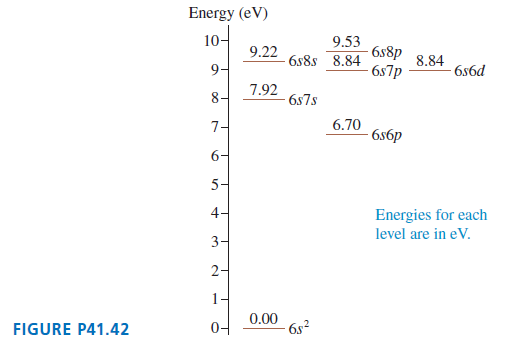
a. Make a table showing all the allowed transitions in the emission spectrum. For each transition, indicate the photon wavelength, in nm.
b. What minimum speed must an electron have to excite the 492-nm-wavelength blue emission line in the Hg spectrum?
Step by Step Solution
There are 3 Steps involved in it
Solve a We need to use the condition I 1 to determine the allowed transitions a... View full answer

Get step-by-step solutions from verified subject matter experts


