(a) Explain why the diffusion coefficient of CH 3 OH is greater than that of sucrose in...
Question:
(a) Explain why the diffusion coefficient of CH3OH is greater than that of sucrose in Table 23-1.
(b) Make an order-of-magnitude estimate of the diffusion coefficient of water vapor in air at 298 K.
Table 23-1
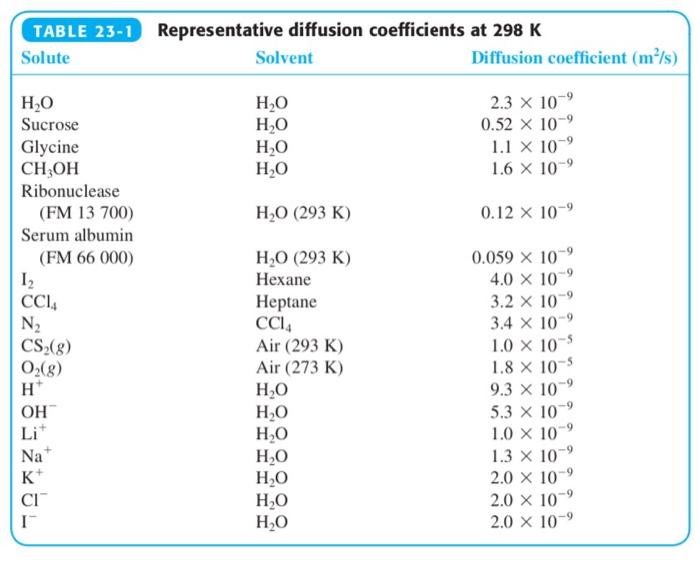
Transcribed Image Text:
TABLE 23-1 Representative diffusion coefficients at 298 K Solute Solvent Diffusion coefficient (m/s) 2.3 x 10 0.52 x 10 H,O H,O H,0 H,O H,O Sucrose Glycine CH,OH 1.1 X 10 1.6 X 10-9 Ribonuclease (FM 13 700) H,O (293 K) 0.12 x 10-9 Serum albumin (FM 66 000) Н.О (293 К) 0.059 X 10 4.0 x 10 3.2 x 10-9 Hexane CCI, N2 CS-(g) O(g) H* Неptane CCI, Air (293 K) Air (273 K) 3.4 x 10 1.0 x 10 1.8 x 10-5 9.3 x 10 5.3 x 10 H,0 H,0 H,0 H,O H,0 H,O H,O OH Li* 1.0 X 10 1.3 x 10 2.0 x 10 2.0 x 10-9 2.0 x 10-9 Na+ K+ CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
a The diffusion coefficient of CH3OH is greater than that of sucrose in Table 231 because CH3OH has ...View the full answer

Answered By

Upasana Nasa
I am a dedicated and hardworking tutor committed to ensuring the success of my students. I am an experienced tutor who has tutored for the last 3 years, which gives me broad experience as a tutor and enable's me to tailor my style to the specific needs of a student. I provide step by step explanation of any problem to enable a student to follow, learn, and understand how to work on the problem on their own.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
a. The diffusion coefficient of sucrose in water at 298 K is 0.522 10 9 m 2 s 1 . Determine the time it will take a sucrose molecule on average to diffuse an rms distance of 1 mm. b. If the molecular...
-
A laboratory apparatus to measure the diffusion coefficient of vapor-gas mixtures consists of a vertical, small-diameter column containing the liquid phase that evaporates into the gas flowing over...
-
The diffusion coefficient of I in hexane at 25C is 4.05 x 10-9 m2 S-1. Estimate the time required for an iodine molecule to have a root mean square displacement of 1.0 cm.
-
The 32-kg spool of outer radius r, = 420 mm has a centroidal radius of gyration k = 265 mm and a central shaft of radius r; = 155 mm. The spool is at rest on the incline when a tension T= 243 N is...
-
How is workforce plans related to business and HR strategies?
-
Prepare a statement of estimated cash receipts and disbursements for October 20X7 for the Botanica Company, which sells one product, herbal soap, by the case. On 1 October 20X7, part of the trial...
-
Is the collision between two billiard balls a nondissipative interaction? (What does the fact that you can hear the collision tell you?)
-
A fuel gas containing methane and ethane is burned with air in a furnace, producing a stack gas at 300C and 105 kPa (absolute). The stack gas contains CO 2 at a partial pressure of 80 mm Hg and no...
-
Looking for figures and an explanation why the NBA's 50/50 revenue split embedded in their CBA helped increase their profits as a league in the long run and why other leagues like the NFL should...
-
An orchard has a acre of specialty fruit trees and is preparing for summer harvest. Historically, this acre has yielded and average of 1,800 lbs of fruit with a standard deviation of 800 lbs. A...
-
Consider the titration of 100.0 mL of 0.010 0 M Ce 4+ in 1 M HClO 4 by 0.040 0 M Cu + to give Ce 3+ and Cu 2+ , using Pt and saturated Ag | AgCl electrodes to find the end point. (a) Write a balanced...
-
A 50.00-mL sample containing La 3+ was treated with sodium oxalate to precipitate La2(C 2 O 4 ) 3 , which was washed, dissolved in acid, and titrated with 18.04 mL of 0.006 363 M KMnO 4 . Write the...
-
The diagram below shows a monopolist's MC and ATC curves as well as the industry demand and MR curves. a. What is the profit-maximizing price and level of output for the monopolist? b. What area in...
-
A source, X = {a, b, c, d), has symbol probabilities {1/3, 1/3,2/9, 1/9}. (a) (10 pts) Use the Huffman algorithm to find an optimal prefix-free code for this source. (b) (10 pts) Is the code {a 00,...
-
Brooke (single, age 35) is a national account director. She earns $125,000 in W-2 salary and another $10,000 in dividends and interest from her brokerage account. She also contributes $20,000 to her...
-
Rent per Leased Square Foot Calculation: Assume that based on your knowledge of the local market, you determine the expected rent is $35 per leasable square foot (LSF) per year. However, you must...
-
An "insect graph" is an unweighted, directed graph resembling an insect in that one vertex - the "body" - appears to have two "antennae" and six "legs". (In the diagram below, there is no particular...
-
Show that divergence of vector A= a, A, +a A, +a_A, in cylindrical coordinate system is developed as: 1 0(rA,) 1 0A, + r V. A= - r +z. rdo dz
-
An astronaut in a rocket moving at 0.50c toward the Sun finds himself halfway between Earth and the Sun. According to the astronaut, how far is he from Earth? In the frame of the Sun, the distance...
-
The pendulum consists of two rods: AB is pin supported at A and swings only in the y-z plane, whereas a bearing at B allows the attached rod BD to spin about rod AB. At a given instant, the rods have...
-
Find the pH of 0.050 M NaCN.
-
Calculate the fraction of association () for 1.00 10-1, 1.00 10-2, and 1.00 1012 M sodium acetate. Does increase or decrease with dilution?
-
A 0.10 M solution of a base has pH = 9.28. Find Kb.
-
Use the standard reduction potentials in the table to create a galvanic cell with the highest possible standard cell voltage. Half-Reaction F,(g) + 2e2F (aq) Cl,(g) + 2e2Cl(aq) Br()+2e2Br (aq)...
-
What is your conclusion about the financial performance of Netflix in the past 3 years (2021 - 2023)?
-
Presume you are investing your life savings in one stock. If there is a 50% chance that the stock would be worthless in one year, what rate of return would you require in order to buy that stock? How...

Study smarter with the SolutionInn App


