Question: Use the standard reduction potentials in the table to create a galvanic cell with the highest possible standard cell voltage. Half-Reaction F,(g) + 2e2F
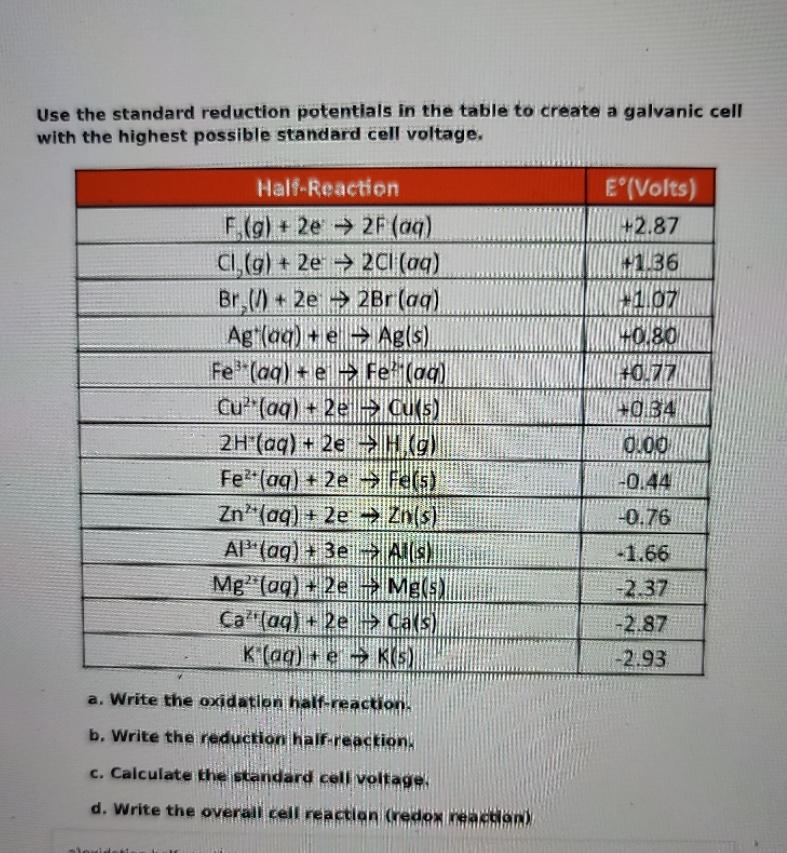
Use the standard reduction potentials in the table to create a galvanic cell with the highest possible standard cell voltage. Half-Reaction F,(g) + 2e2F (aq) Cl,(g) + 2e2Cl(aq) Br()+2e2Br (aq) E(Volts) +2.87 +1.36 +1.07 Ag (aq) + e Ag(s) +0.80 Fe (aq) eFe (aq) +0.77 Cu (aq) + 2e CU(s) +0.34 2H (aq) + 2e H(g) 0.00 Fe (aq) + 2e Fe(s) -0.44 Zn (aq) + 2e Zn(s) -0.76 Al (aq) +3eA(s) -1.66 Mg (aq) + 2e Mg(s) -2.37 Ca (aq) + 2eCa(s) -2.87 K(aq) +eK(5) -2.93 a. Write the oxidation half-reaction. b. Write the reduction half-reaction. c. Calculate the standard cell voltage. d. Write the overall cell reaction (redox reaction) aloxidati
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


