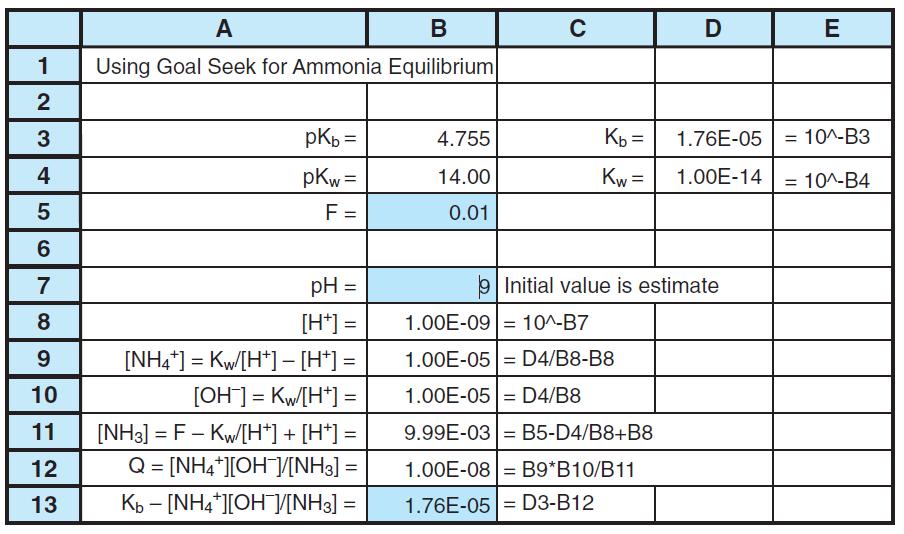
Modify Figure 8-7 to find the concentrations of species in 0.05 M NH3. The only change required
Question:
Modify Figure 8-7 to find the concentrations of species in 0.05 M NH3. The only change required is the value of F. How do the pH and fraction of ammonia hydrolysis (= [NH+4 ]/([NH+4 ] + [NH3])) change when the formal concentration of NH3 increases from 0.01 to 0.05 M?
Transcribed Image Text:
А В C E Using Goal Seek for Ammonia Equilibrium pKb = 4.755 Kb = 1.76E-05 = 10^-B3 4 pKw = 14.00 Kw = 1.00E-14 10^-B4 %3D F = 0.01 7 pH = Initial value is estimate 8 [H*] = 1.00E-09 10^-B7 9. [NH4*] = Kw/[H*] - [H*] = 1.00E-05= D4/B8-B8 10 [OH] = Kw[H*] = 1.00E-05 = D4/B8 [NH3] = F - Kw[H*] + [H*] = Q = [NH4*][OHV[NH3] = 11 9.99E-03 = B5-D4/B8+B8 12 1.00E-08 = B9*B10/B11 %3D 13 Kb - [NH4*][OH]/V[NH3] = 1.76E-05= D3-B12 2. LO
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 41% (12 reviews)
pH changes from 10 to 9 at the equivalence point Fraction of NH3 hydrolysis changes from 06001 to 04...View the full answer

Answered By

Enock Oduor
I am a chemist by profession, i coach high school students with their homework, i also do more research during my free time, i attend educational and science fair seminars where i meet students and do some projects.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
(a) Find the concentrations of species in saturated CaF 2 as a function of pH by using Reactions 12-32 through 12-36 and adding the following reaction: Do not include activity coefficients. Produce a...
-
Find the concentrations of Cu2+ (aq), NH3 (aq), and [Cu(NH3)4]2+(aq) at equilibrium when 0.10 mol Cu2+(aq) and 0.40 mol NH3(aq) are made up to 1.00 L of solution. The dissociation constant, Kd, for...
-
Find the concentrations of Ag+(aq), NH3(aq), and [Ag(NH3)2]+(aq) at equilibrium when 0.10 mol Ag+(aq) and 0.10 mol NH3(aq) are made up to 1.00 L of solution. The dissociation constant, Kd, for the...
-
The University of Professional Studies, Accra (UPSA) is a public university in Ghana. UPSA is the first university in Ghana to provide both academic and business professional education. The...
-
Repeat Exercise 1b, substituting respondent's social class (CLASS) as the independent variable in separate models for men and women. What can you conclude about the relationship between CLASS and...
-
Google went public in August 2004 at $85, a price regarded by many at the time as ridiculously high. By May of 2005, the price had tripled. By late 2007, the price was over $600 a share but fell...
-
Which of the following items could cause the recognition of accrued liabilities? (a) Sales, interest expense, rent. (b) Sales, taxes, interest income. (c) Salaries, rent, insurance. (d) Salaries,...
-
The stockholder's equity accounts of Pen Corporation and Sin Corporation at December 31, 2010, were as follows (in thousands) On January 1, 2011, Pen Corporation acquired an 80 percent interest in...
-
1. Use the following information to solve parts a-f: Returns State Prob AS BS Boom 0.1 0.25 0.18 Growth 0.2 0.10 0.20 Normal 0.5 0.15 0.04 Recession 0.2 -0.12 0.00 a. What is the expected return for...
-
The Spartans Company has the capacity to produce 369,000 t-shirts per year and is currently selling 344,000 t-shirts for $9 each. The variable cost associated with each t-shirt is $7.75. A retailer...
-
A 40.0-mL solution of 0.040 0 M Hg 2 (NO 3 ) 2 was titrated with 60.0 mL of 0.100 M KI to precipitate Hg 2 I 2 (K sp = 4.6 10 -29 ). (a) Show that 32.0 mL of KI are needed to reach the equivalence...
-
Find the concentrations of the major species in a saturated aqueous solution of LiF. Consider these reactions: (a) Look up the equilibrium constants in the appendixes and write their pK values. The...
-
What is a mutual fund? What are the two ways in which you earn a return on a mutual fund investment?
-
ABC Company, a public organization, engages K. Young, CPA, to audit their financial statements. In this scenario, the intended users of the financial statements would be Select answer from the...
-
Tommy purchased a new clean vehicle in 2023. What is the amount of the Clean Vehicle Tax Credit, assuming the transaction otherwise qualifies for the tax credit?
-
What do you believe based on the textbook's overview on how to manage social media information, is the best approach and why? What do you believe blockchain can do to social media company's best...
-
explain, To encourage citizens to pay taxes willingly, it's crucial for tax systems to rely on mass voluntary compliance, as it's expensive to collect taxes through detection and punishment. Some...
-
You are managing a multi-year project whose budget is 500,000 USD. The cost variance is -45,000 USD, the actual cost is 200,000 USD, and the planned value is 140,000 USD. Calculate the schedule...
-
The first two dark fringes on one side of the central maximum in a single-slit diffraction pattern are 1.0 mm apart. The wavelength of the light is 610 nm and the screen is 1.0 m from the slit. What...
-
Burberrys competitive advantage is through its differentiation strategy. What risk should Burberry remain aware of?
-
What does the selectivity coefficient tell us? Is it better to have a large or a small selectivity coefficient?
-
Suppose that the silver-silver chloride electrode in Figure 14-2 is replaced by a saturated calomel electrode. Calculate the cell voltage if [Fe 2+ ] / [Fe 3+ ] = 2.5 10 -3 . Figure 14-2
-
Why is it preferable to use a metal ion buffer to achieve pM = 8 rather than just dissolving enough M to give a 10-8 M solution?
-
$70 $60 $50 $ per unit a Private MC d $40 C social MB e private MB 0 #of units 20 36 55 The graph above shows the market for front-yard trees. What price will allow this market to reach the socially...
-
The US government is aware of the economy's sensitivity to supply chain issues and has initiated a response. Using your own independent research, explain the actions the US government has taken to...
-
Suppose that between 1995 and 2013, US external that rose from a percent of GDP to over 29% of GDP this increase in external debt owed to foreign entities ?Explain

Study smarter with the SolutionInn App


