Question: The figure shows reversed-phase retention data for three compounds. a. Identify whether compounds A, B, and C are weak acids or bases. For each compound,
The figure shows reversed-phase retention data for three compounds.
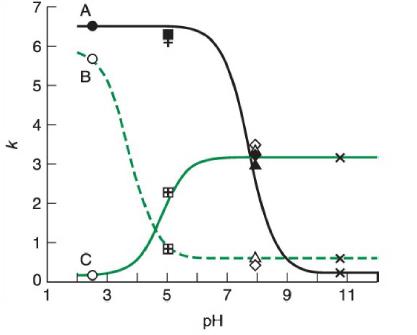
a. Identify whether compounds A, B, and C are weak acids or bases. For each compound, what is the pKapKa and the retention factor of the more-retained form?
b. Over what pHpH range would a method be least rugged with regard to retention of component C?
c. Each different symbol in the plot indicates a different buffer (circle=pH 2.48 circle=pH 2.48 phosphate; plus=pH 5.01 plus = pH 5.01 acetate; and so on). Why are different buffers used for this experiment?
k 7 6 5 4 3 2 1 0 1 A C 3 5 7 PH 9 11
Step by Step Solution
3.47 Rating (160 Votes )
There are 3 Steps involved in it
a To determine whether compounds A B and C are weak acids or bases we need to look at their behavior ... View full answer

Get step-by-step solutions from verified subject matter experts


