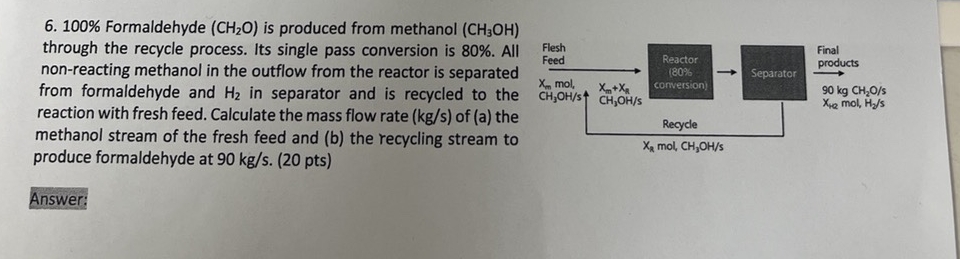
Question: 1 0 0 % Formaldehyde ( C H 2 O ) is produced from methanol ( C H 3 O H ) through the recycle
Formaldehyde is produced from methanol through the recycle process. Its single pass conversion is All nonreacting methanol in the outflow from the reactor is separated from formaldehyde and in separator and is recycled to the reaction with fresh feed. Calculate the mass flow rate of a the methanol stream of the fresh feed and b the recycling stream to produce formaldehyde at pts
Answer:
Formaldehyde is produced from methanol through the recycle process. Its single pass conversion is All nonreacting methanol in the outflow from the reactor is separated from formaldehyde and in separator and is recycled to the reaction with fresh feed. Calculate the mass flow rate of a the methanol stream of the fresh feed and b the recycling stream to produce formaldehyde at
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


