Question: 1 1 . 1 1 . 2 0 2 4 Thermodynamics 1 Homework Gas 1 of mass m 1 , at first at P 1
Thermodynamics Homework
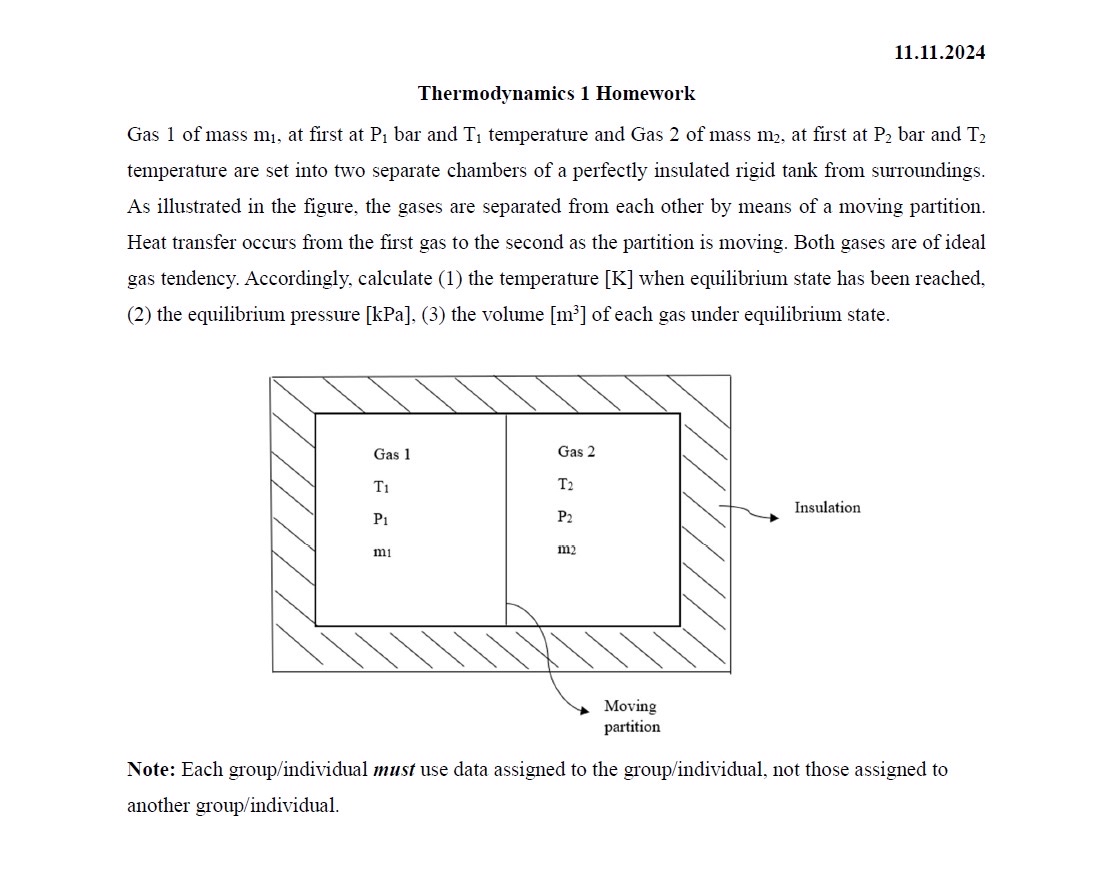
Gas of mass at first at bar and temperature and Gas of mass at first at bar and temperature are set into two separate chambers of a perfectly insulated rigid tank from surroundings. As illustrated in the figure, the gases are separated from each other by means of a moving partition. Heat transfer occurs from the first gas to the second as the partition is moving. Both gases are of ideal gas tendency. Accordingly, calculate the temperature K when equilibrium state has been reached, the equilibrium pressure kPa, the volume of each gas under equilibrium state.
Note: Each groupindividual must use data assigned to the groupindividual not those assigned to another groupindividual
Gas: oxygen
Gas: Nitrogen
TK
TK
Pkpa
Pkpa
Mkg
Mkg
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


