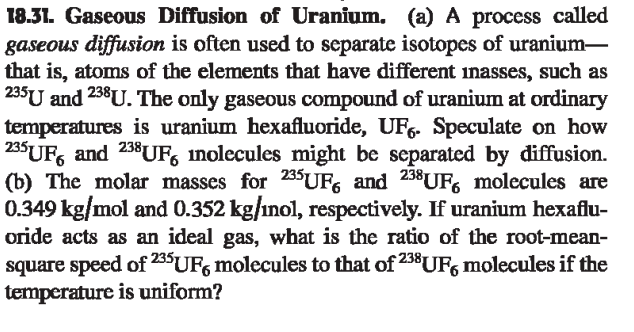
Question: 1 8 . 3 1 . Gaseous Diffusion of Uranium. ( a ) A process called gaseous diffusion is often used to separate isotopes of
Gaseous Diffusion of Uranium. a A process called
gaseous diffusion is often used to separate isotopes of uranium
that is atoms of the elements that have different inasses, such as
and The only gaseous compound of uranium at ordinary
temperatures is uranium hexafluoride, Speculate on how
and inolecules might be separated by diffusion.
b The molar masses for and molecules are
and nol, respectively. If uranium hexaflu
oride acts as an ideal gas, what is the ratio of the rootmean
square speed of molecules to that of molecules if the
temperature is uniform?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


