Question: = 1. A certain reaction has the ff. mechanism: A + A 2 A* + A, ki = forward rxn, k, = backward rxn =
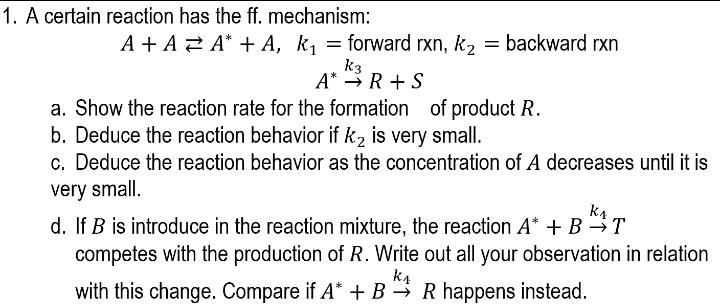
= 1. A certain reaction has the ff. mechanism: A + A 2 A* + A, ki = forward rxn, k, = backward rxn = A k2 = k3 A* R+S a. Show the reaction rate for the formation of product R. b. Deduce the reaction behavior if kz is very small. c. Deduce the reaction behavior as the concentration of A decreases until it is very small. d. If B is introduce in the reaction mixture, the reaction A* + B T B kat competes with the production of R. Write out all your observation in relation k4 with this change. Compare if A* + B R happens instead
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


