Question: 1. a) Given the information contained in the attached Ellingham diagram, calculate the molar change in free energy for chromium oxidation reaction at 300 C
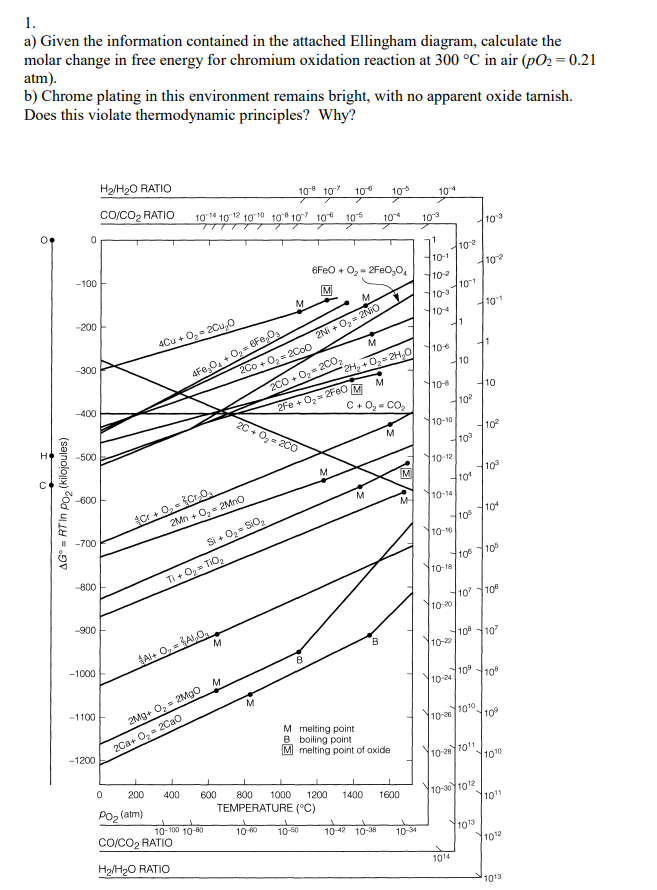
1. a) Given the information contained in the attached Ellingham diagram, calculate the molar change in free energy for chromium oxidation reaction at 300 C in air (pO2 = 0.21 atm). b) Chrome plating in this environment remains bright, with no apparent oxide tarnish. Does this violate thermodynamic principles? Why? H2/H20 RATIO 10- 107 10" 102 103 104 CO/CO2 RATIO 101 102 103 108 107 106 105 104 10-3 10-3 0 0 1 110-2 10-1 102 6FeO +0,- 2FeO,04 10-2 -100 101 M 10-3 104 10-1 -200 11 2N + 0,2NO 404 +,200,0 41 10-6 10 -300 4F6,0, +0, F8,0 2Co + 0,2CoO M 10-8 10 200+ O, 2002 M 2H + O2H,0 2Fe + 0,2FeO M 102 C+0,-CO, -400 10-10 102 M O, 200 100 H -500 710-12 M 100 M 104 C M 10-14 AG = RT in Poz (kilojoules) -600 M 4104 4105 C+0. sto 2Mn + 0,2Mno 10-16 -700 7106 10 Si + O, Sio Ti +O, TIO 10-18 800 4107 10 10-20 -900 10 10 M B 10-22 $A+O, ALO. B -1000 10 10% M 10-24 -1100 110-100 10 Mg+ O, 2MgO 2Ca+ 0 = 2Cao M melting point B boiling point M melting point of oxide -1200 10-281011 100 10-301012 1011 1 0 200 400 600 800 1000 1200 1400 1600 TEMPERATURE (C) 10-100 10 80 10-6 10-50 102 103 CO/CO2 RATIO Poz(atm) 10-30 103 1012 1014 Hy/H2O RATIO 1013
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


