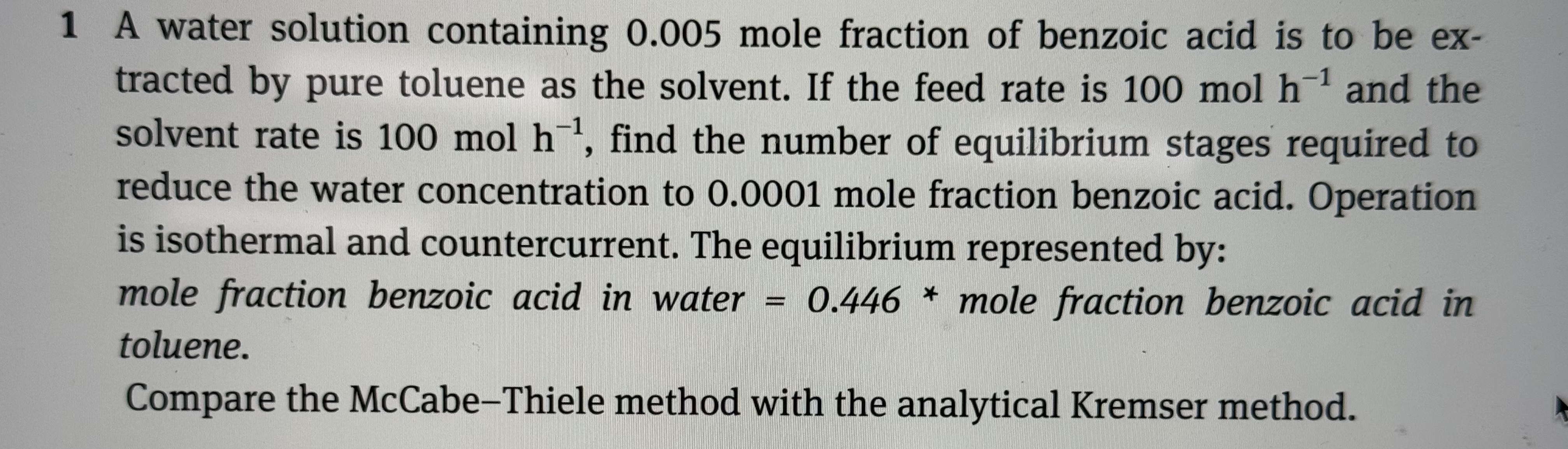
Question: 1 A water solution containing 0 . 0 0 5 mole fraction of benzoic acid is to be extracted by pure toluene as the solvent.
A water solution containing mole fraction of benzoic acid is to be extracted by pure toluene as the solvent. If the feed rate is and the solvent rate is find the number of equilibrium stages required to reduce the water concentration to mole fraction benzoic acid. Operation is isothermal and countercurrent. The equilibrium represented by: mole fraction benzoic acid in water mole fraction benzoic acid in toluene.
Compare the McCabeThiele method with the analytical Kremser method.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


