Question: 1- Determine the cell notation for the redox reaction given below. Sn(s)+2Ag+(aq)Sn2+(aq)+2Ag(s) a) Ag+(aq)Ag(s)Sn(s)Sn2+(aq), b) Sn (s) Sn2+ (aq) Ag+(aq) Ag (s), c) Ag(s)Ag+(aq)Sn2+(aq)Sn(s) d)
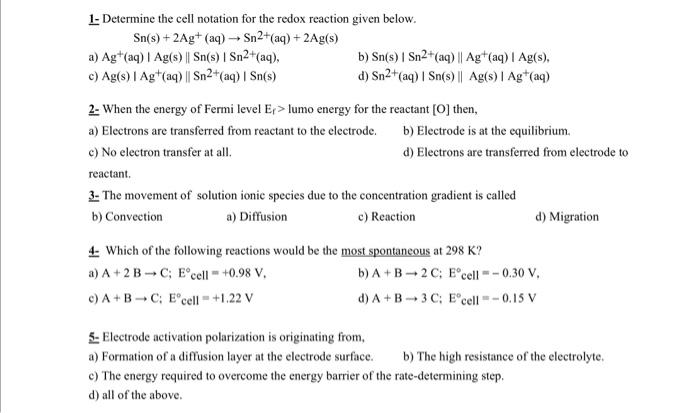
1- Determine the cell notation for the redox reaction given below. Sn(s)+2Ag+(aq)Sn2+(aq)+2Ag(s) a) Ag+(aq)Ag(s)Sn(s)Sn2+(aq), b) Sn (s) Sn2+ (aq) Ag+(aq) Ag (s), c) Ag(s)Ag+(aq)Sn2+(aq)Sn(s) d) Sn2+(aq)Sn(s)Ag(s)Ag+(aq) 2- When the energy of Fermi level Ef> lumo energy for the reactant [O] then, a) Electrons are transferred from reactant to the electrode. b) Electrode is at the equilibrium. c) No electron transfer at all. d) Electrons are transferred from electrode to reactant. 3- The movement of solution ionic species due to the concentration gradient is called b) Convection a) Diffusion c) Reaction d) Migration 4- Which of the following reactions would be the most spontaneous at 298K ? a) A+2BC;E cell =+0.98V b) A+B2C; E cell =0.30V, c) A+BC:E cell =+1.22V d) A+B3C;E cell =0.15V 5- Electrode activation polarization is originating from, a) Formation of a diffusion layer at the electrode surface. b) The high resistance of the electrolyte. c) The energy required to overcome the energy barrier of the rate-determining step. d) all of the above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


