Question: 1. erfc diffusion One surface of a 12 mm thick 0.01 wt% C steel plate (p = 7780 kg/m) at 1000C is brought into
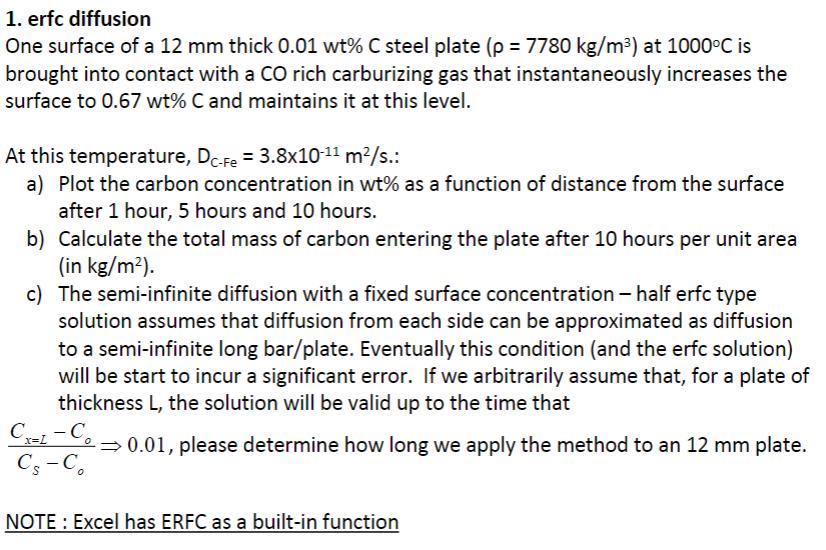
1. erfc diffusion One surface of a 12 mm thick 0.01 wt% C steel plate (p = 7780 kg/m) at 1000C is brought into contact with a CO rich carburizing gas that instantaneously increases the surface to 0.67 wt% C and maintains it at this level. At this temperature, DC-Fe = 3.8x10-11 m/s.: a) Plot the carbon concentration in wt% as a function of distance from the surface after 1 hour, 5 hours and 10 hours. b) Calculate the total mass of carbon entering the plate after 10 hours per unit area (in kg/m). c) The semi-infinite diffusion with a fixed surface concentration half erfc type solution assumes that diffusion from each side can be approximated as diffusion to a semi-infinite long bar/plate. Eventually this condition (and the erfc solution) will be start to incur a significant error. If we arbitrarily assume that, for a plate of thickness L, the solution will be valid up to the time that Cx=L-Co CS-Co 0.01, please determine how long we apply the method to an 12 mm plate. NOTE: Excel has ERFC as a built-in function
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


