Question: 1. For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that would occur between the
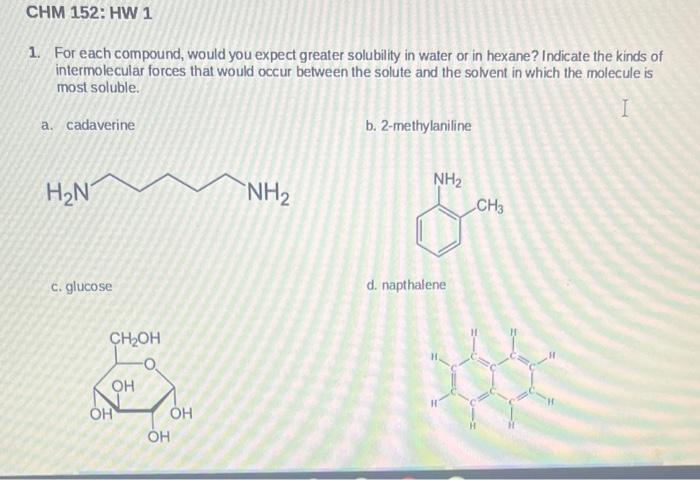
1. For each compound, would you expect greater solubility in water or in hexane? Indicate the kinds of intermolecular forces that would occur between the solute and the solvent in which the molecule is most soluble. a. cadaverine b. 2-methylaniline c. glucose d. napthalene
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


