Question: 1) Formulate the resonance structures for the following compounds. Use curved arrows to indicate the movement of electrons and indicate what you think is the
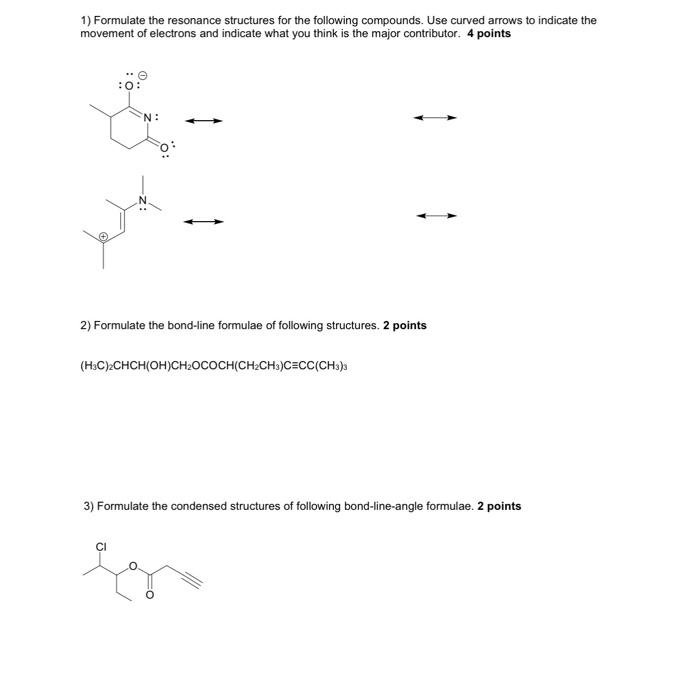
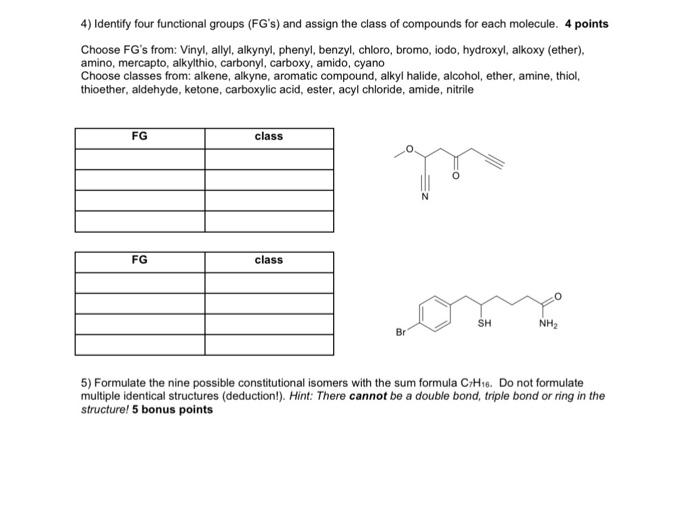
1) Formulate the resonance structures for the following compounds. Use curved arrows to indicate the movement of electrons and indicate what you think is the major contributor. 4 points 2) Formulate the bond-line formulae of following structures. 2 points (H3C)2CHCH(OH)CH2OCOCH(CH2CH3)CCC(CH3)3 3) Formulate the condensed structures of following bond-line-angle formulae. 2 points 4) Identify four functional groups (FG's) and assign the class of compounds for each molecule. 4 points Choose FG's from: Vinyl, allyl, alkynyl, phenyl, benzyl, chloro, bromo, iodo, hydroxyl, alkoxy (ether), amino, mercapto, alkylthio, carbonyl, carboxy, amido, cyano Choose classes from: alkene, alkyne, aromatic compound, alkyl halide, alcohol, ether, amine, thiol, thioether, aldehyde, ketone, carboxylic acid, ester, acyl chloride, amide, nitrile 5) Formulate the nine possible constitutional isomers with the sum formula C7H16. Do not formulate multiple identical structures (deduction!). Hint: There cannot be a double bond, triple bond or ring in the structure! 5 bonus points
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


