Question: organic chemistry help Class Activity 2 Resonance Structures Model 3: Stability of Resonance Forms Not all resonance forms have the same energy, some forms may
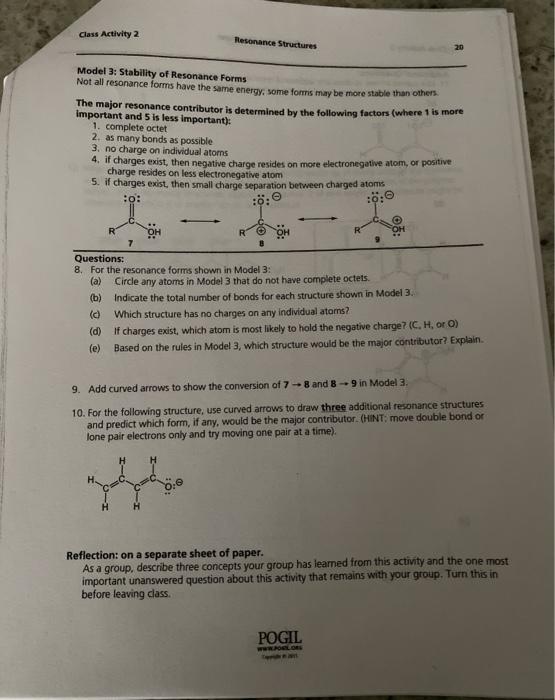
Class Activity 2 Resonance Structures Model 3: Stability of Resonance Forms Not all resonance forms have the same energy, some forms may be more stable than others The major resonance contributor is determined by the following factors (where 1 is more Important and 5 is less important): 1. complete octet 2. as many bonds as possible 3. no charge on individual atoms 4. if charges exist, then negative charge resides on more electronegative atom, or positive charge resides on less electronegative atom 5. if charges exist, then small charge separation between charged atoms OH 7 Questions: 8. For the resonance forms shown in Model 3: (a) Circle any atoms in Model 3 that do not have complete octets. (b) indicate the total number of bonds for each structure shown in Model 3. id Which structure has no charges on any individual ators? (d) If charges exist, which atom is most likely to hold the negative charge? (C. Horo) (e) Based on the rules in Model 3, which structure would be the major contributor? Explain. 9. Add curved arrows to show the conversion of 7 - 8 and 8 - 9 in Model 3 10. For the following structure, use curved arrows to draw three additional resonance structures and predict which form, if any, would be the major contributor. (HINT: move double bond or lone pair electrons only and try moving one pair at a time) Reflections on a separate sheet of paper. As a group, describe three concepts your group has learned from this activity and the one most important unanswered question about this activity that remains with your group. Turn this in before leaving class POGIL
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


