Question: 1) Gas A dissolves in liquid B in a beaker and diffuses isothermally into the liquid phase as shown in the figure. As it diffuses,
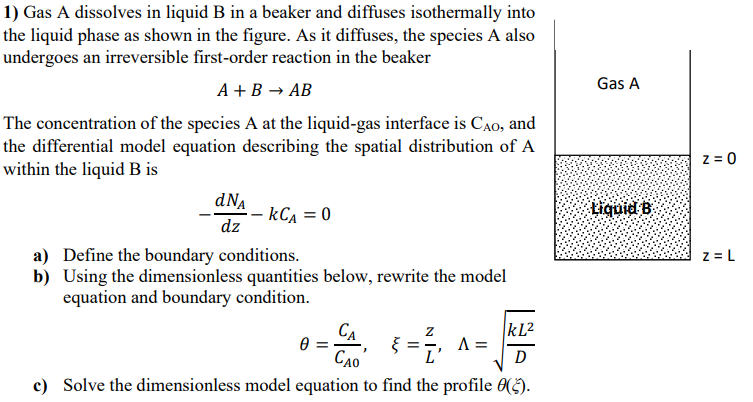
1) Gas A dissolves in liquid B in a beaker and diffuses isothermally into the liquid phase as shown in the figure. As it diffuses, the species A also undergoes an irreversible first-order reaction in the beaker A+BAB The concentration of the species A at the liquid-gas interface is CAO, and the differential model equation describing the spatial distribution of A within the liquid B is dzdNAkCA=0 a) Define the boundary conditions. b) Using the dimensionless quantities below, rewrite the model equation and boundary condition. =CA0CA,=Lz,=DkL2 c) Solve the dimensionless model equation to find the profile ()
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


