Question: Gas A dissolves into liquid B in a beaker and diffuses isothermally into the liquid phase. As it diffuses, A also undergoes an irreversible first-order
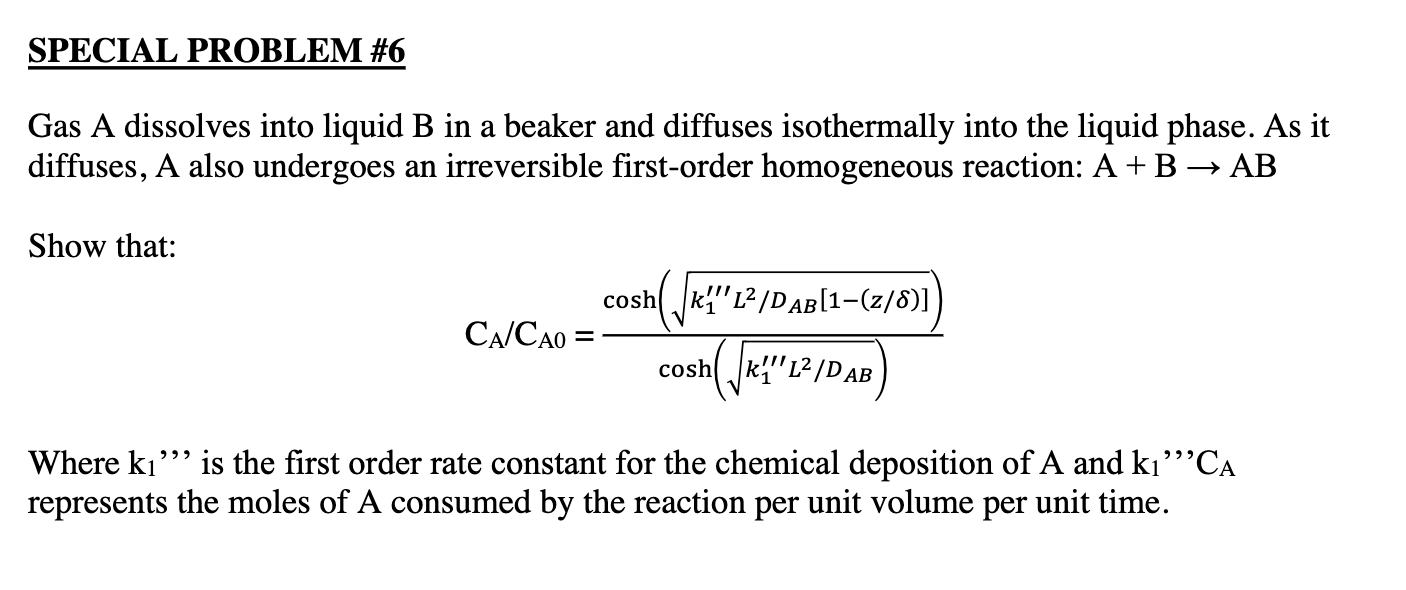
Gas A dissolves into liquid B in a beaker and diffuses isothermally into the liquid phase. As it diffuses, A also undergoes an irreversible first-order homogeneous reaction: A+BAB Show that: CA/CA0=cosh(k1L2/DAB)cosh(k1L2/DAB[1(z/)]) Where k1,, is the first order rate constant for the chemical deposition of A and k1,,CA represents the moles of A consumed by the reaction per unit volume per unit time. Gas A dissolves into liquid B in a beaker and diffuses isothermally into the liquid phase. As it diffuses, A also undergoes an irreversible first-order homogeneous reaction: A+BAB Show that: CA/CA0=cosh(k1L2/DAB)cosh(k1L2/DAB[1(z/)]) Where k1,, is the first order rate constant for the chemical deposition of A and k1,,CA represents the moles of A consumed by the reaction per unit volume per unit time
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


