Question: 1. (50%) Gas A dissolves in liquid B in a beaker and diffuses isothermally into the liquid phase. As it diffuse, gas A also undergoes
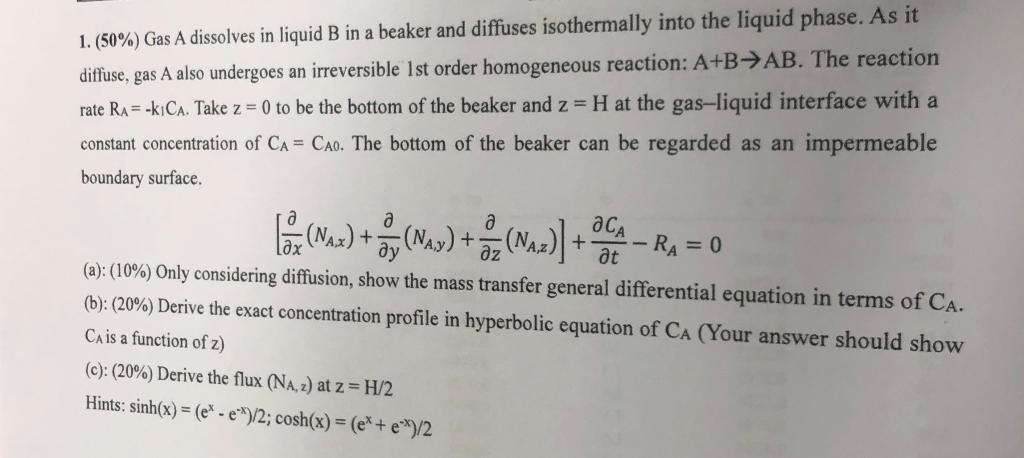
1. (50\%) Gas A dissolves in liquid B in a beaker and diffuses isothermally into the liquid phase. As it diffuse, gas A also undergoes an irreversible 1st order homogeneous reaction: A+BAB. The reaction rate RA=k1CA. Take z=0 to be the bottom of the beaker and z=H at the gas-liquid interface with a constant concentration of CA=CA0. The bottom of the beaker can be regarded as an impermeable boundary surface. [x(NA,x)+y(NA,y)+z(NA,z)]+tCARA=0 (a): (10\%) Only considering diffusion, show the mass transfer general differential equation in terms of CA. (b): (20\%) Derive the exact concentration profile in hyperbolic equation of CA (Your answer should show CA is a function of z ) (c): (20\%) Derive the flux (NA,z) at z=H/2 Hints: sinh(x)=(exex)/2;cosh(x)=(ex+ex)/2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


