Question: When iron metal reacts with sulfuric acid, it produces iron (III) sulfate and hydrogen gas. Balance the equation: Fe + H2SO4 Fe2(SO4)3 + H2
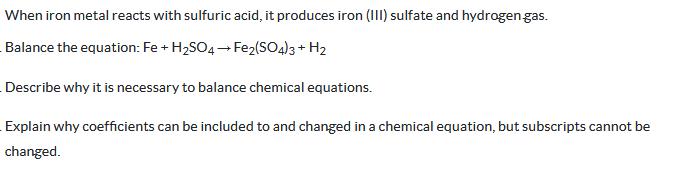
When iron metal reacts with sulfuric acid, it produces iron (III) sulfate and hydrogen gas. Balance the equation: Fe + H2SO4 Fe2(SO4)3 + H2 Describe why it is necessary to balance chemical equations. Explain why coefficients can be included to and changed in a chemical equation, but subscripts cannot be changed.
Step by Step Solution
3.35 Rating (158 Votes )
There are 3 Steps involved in it
The balanced chemical equation for the reaction between iron metal and sulfuric acid is Fe HSO FeSO H Heres a breakdown Iron Fe We have 1 iron atom on ... View full answer

Get step-by-step solutions from verified subject matter experts


