Question: 1. Select the compound that can be prepared using acetoacetic acid synthesis? 2. What is the product? 3. What is the product? 4. Show the
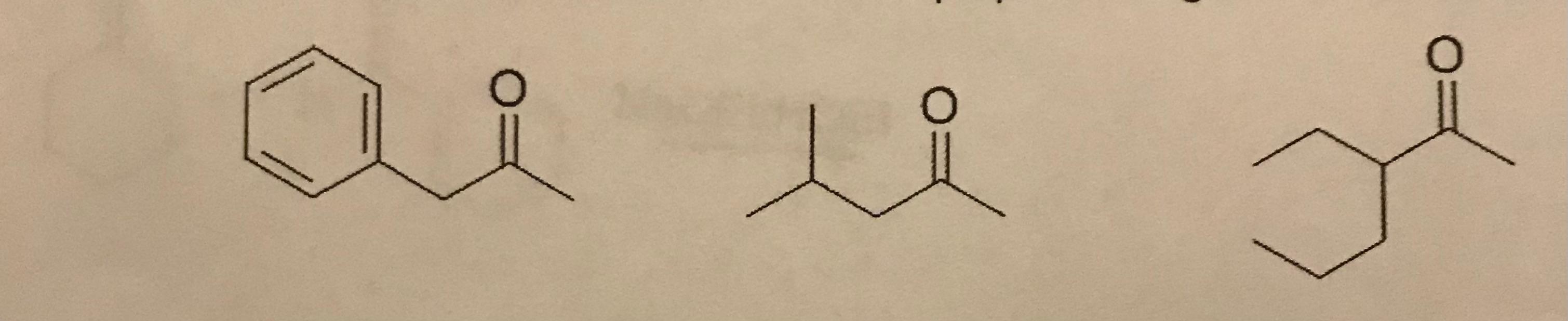
1. Select the compound that can be prepared using acetoacetic acid synthesis?
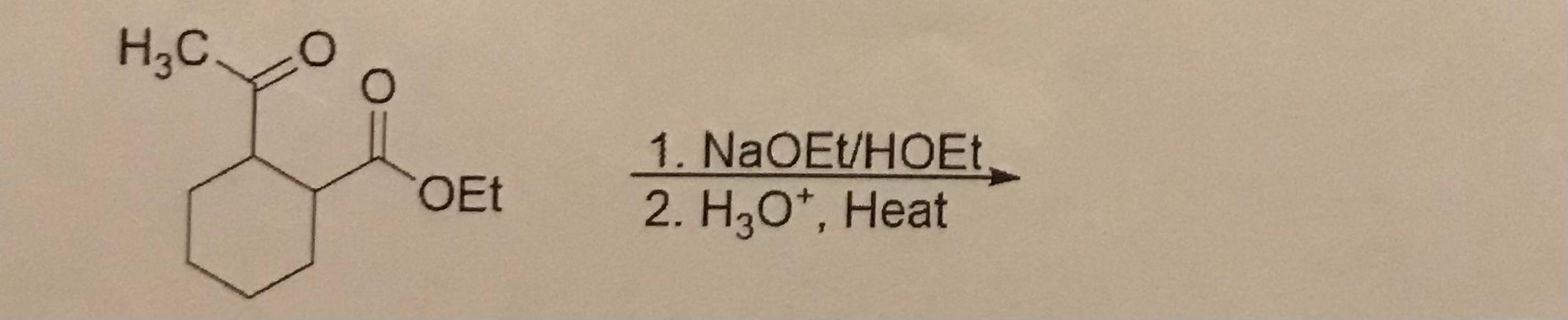
2. What is the product?
3. What is the product?
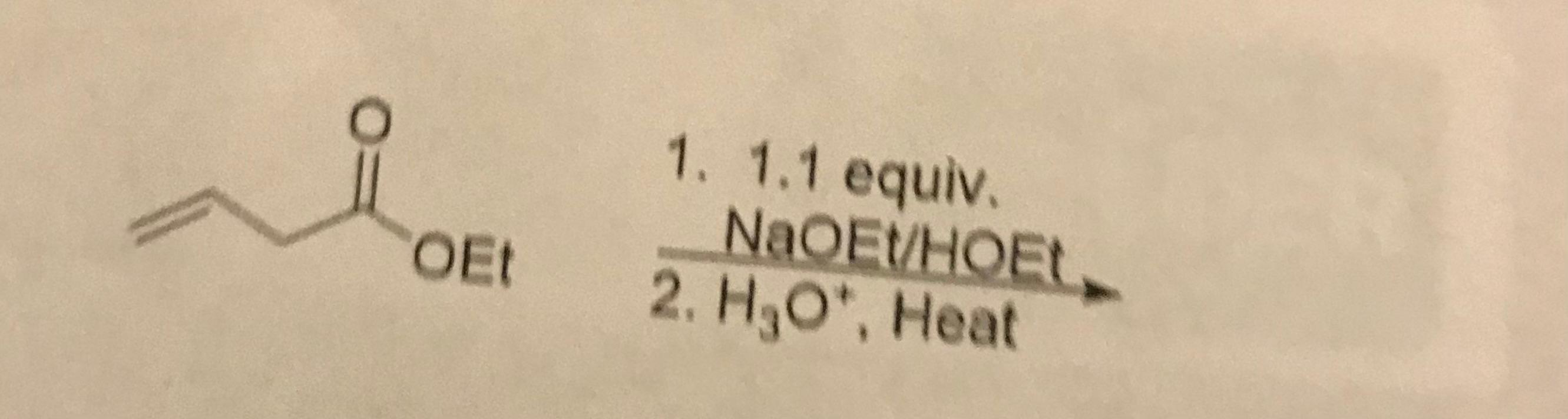
4. Show the mechanism including all intermediates
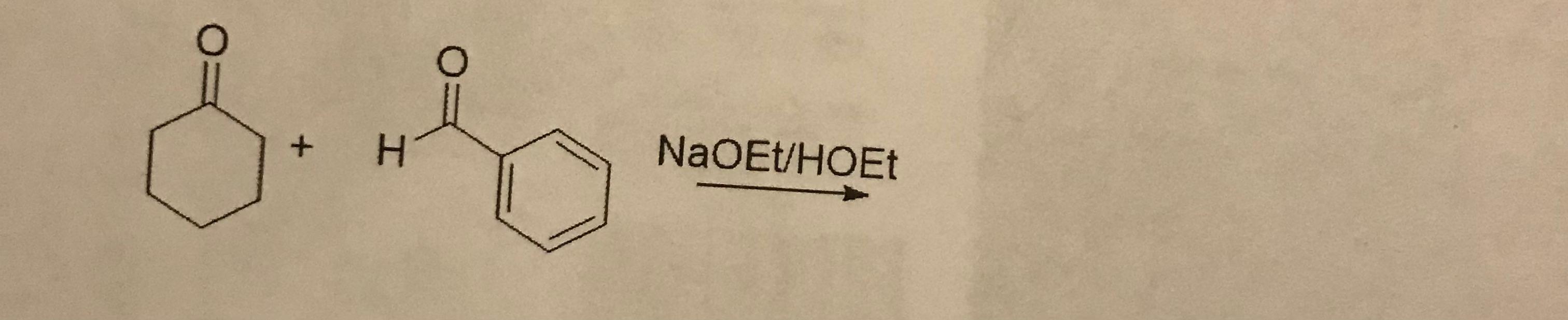
5. What is the product?
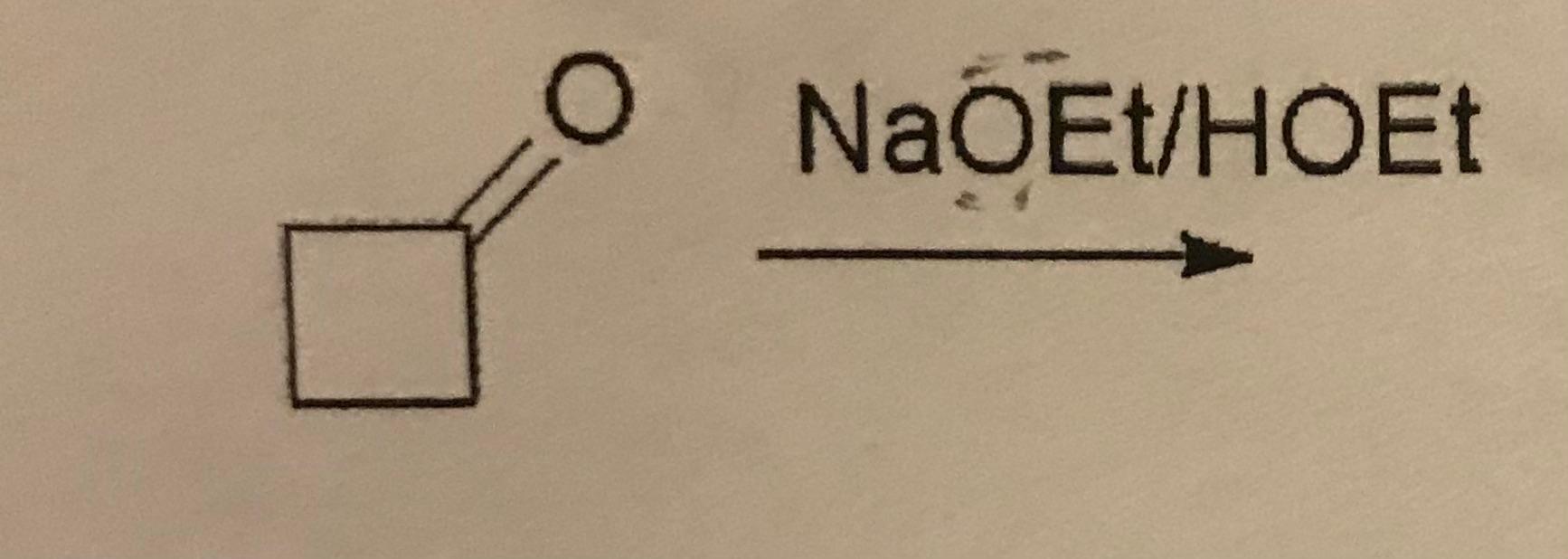
6. What is the product?
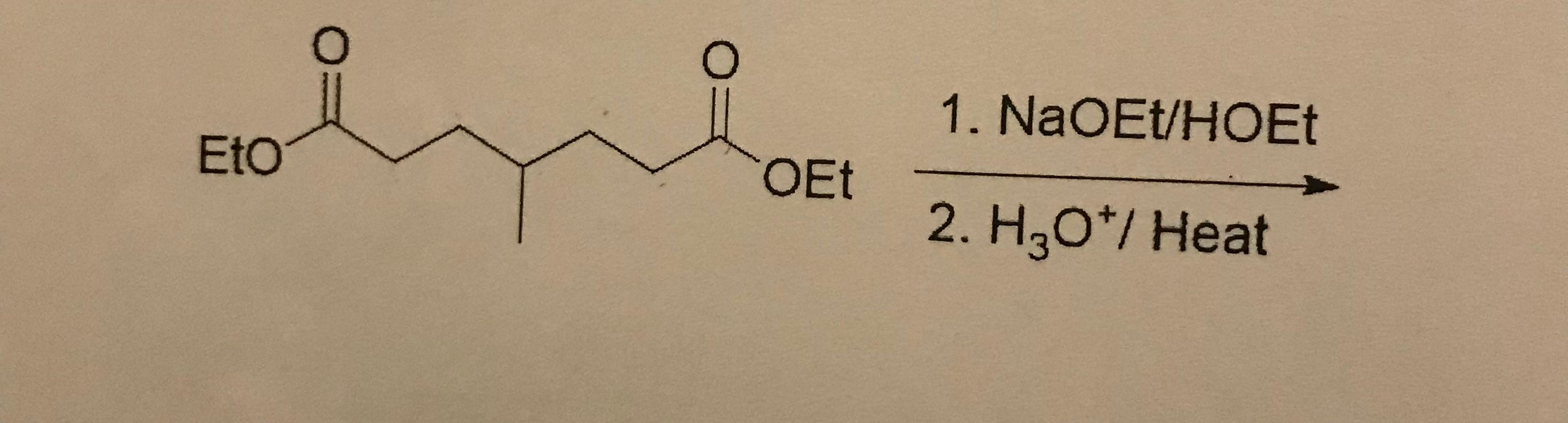
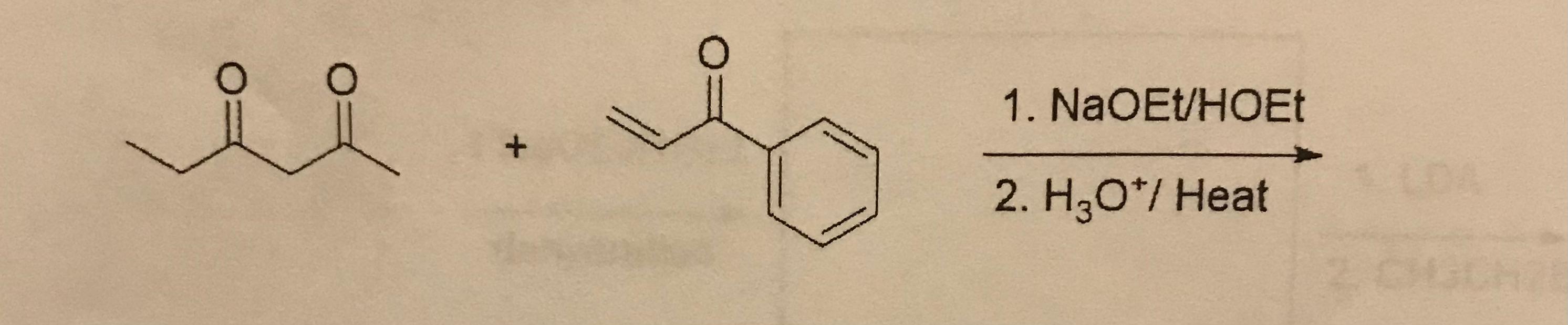
7. What is the full Robinson Annulation product including all intermediates?
8. Show mechanism including all intermediates
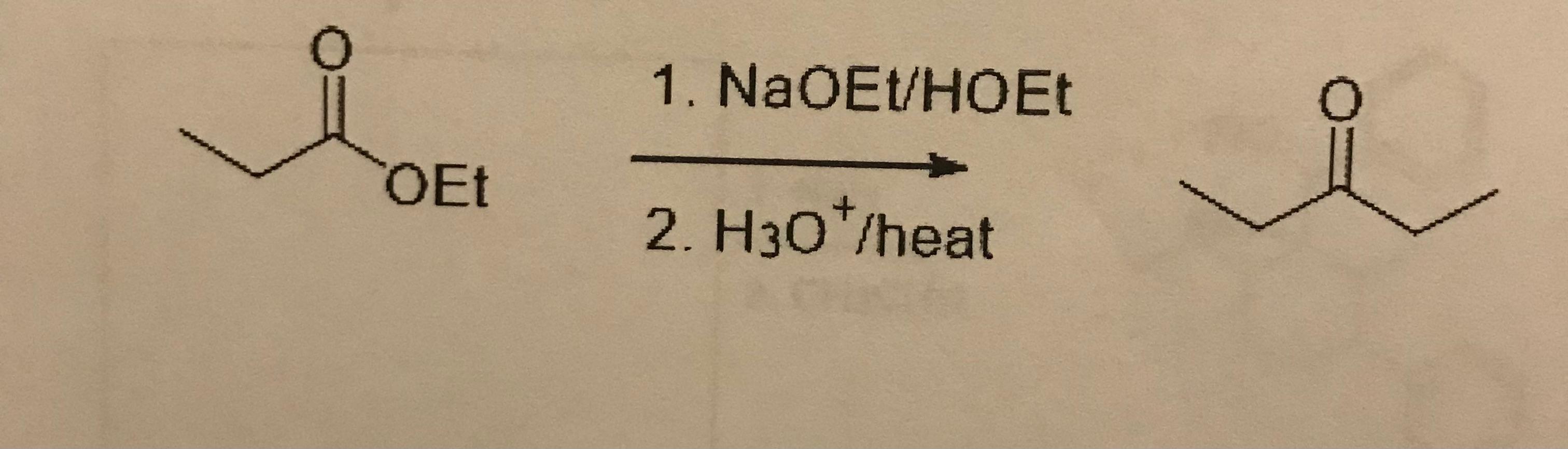
9. Fill in the products for each step
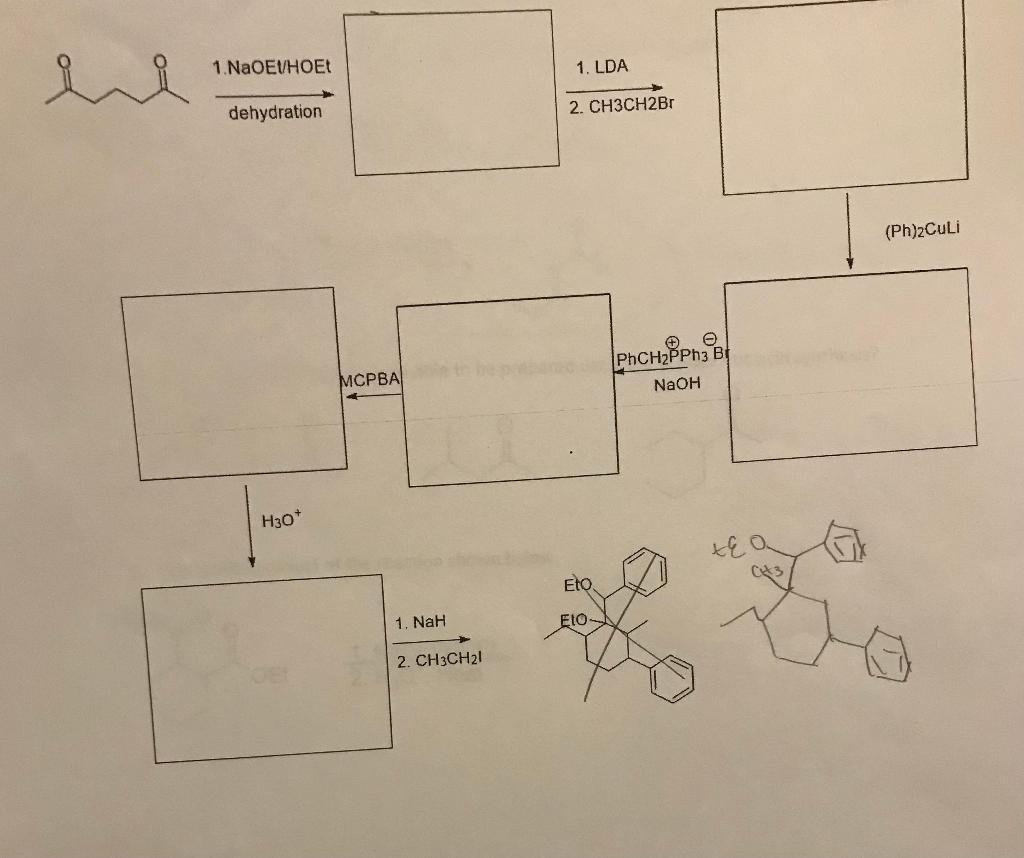
O Qui ui . O OEt 1. NaOELHOE 2. H30*, Heat OET 1. 1.1 equiv. NaoFt/HOET 2. H,O*Heat Bry + NaOELHOET O Nao Et/HOE O 0 1. NaOET/HOEt Eto OEt 2. H2O+/ Heat 0 i 1. NaOEU/HOEt + 2. H20*/ Heat 1. NaOEU/HOEt OEt 2. H307/heat 1. NaOEVHOE 1. LDA dehydration 2. CH3CH2Br (Ph)2Culi o PhCH2PPh3 B NaOH MCPBA H30* Eto 1. Nah ELO 2. CH3CH21
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


