Question: 1. The graph below belongs to a titration between a. HClO4(aq) with NaOH(aq) b. CH3COOH(aq) with NaOH(aq) c. HCN(aq) with NaOH(aq) d. H2S(aq) with NaOH(aq)
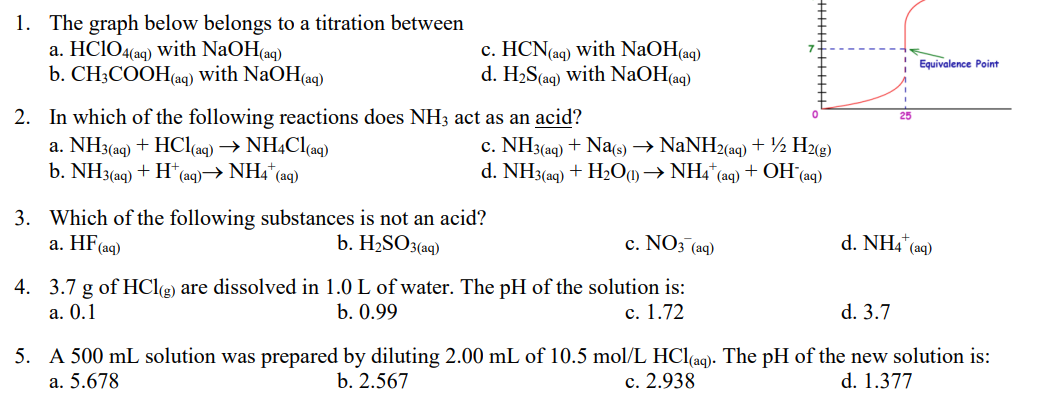
1. The graph below belongs to a titration between a. HClO4(aq) with NaOH(aq) b. CH3COOH(aq) with NaOH(aq) c. HCN(aq) with NaOH(aq) d. H2S(aq) with NaOH(aq) Equivalence Point 2. In which of the following reactions does NH3 act as an acid? a. NH3(aq) + HCl(aq) + NH4Cl(aq) c. NH3(aq) + Nas) NaNH2(aq) + 12 H2(g) b. NH3(aq) + H+ (aq) NH4+ (aq) d. NH3(aq) + H2O1) NH4+ (aq) + OH (aq) + 3. Which of the following substances is not an acid? a. HF (aq) b. H2SO3(aq) c. NO3(aq) d. NH4(aq) a. 0.1 4. 3.7 g of HCl(g) are dissolved in 1.0 L of water. The pH of the solution is: b. 0.99 c. 1.72 d. 3.7 5. A 500 mL solution was prepared by diluting 2.00 mL of 10.5 mol/L HCl(aq). The pH of the new solution is: a. 5.678 b. 2.567 c. 2.938 d. 1.377
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


