Question: 1. The temperature and conversion in a very long (i.e., virtually infinite) PFR are shown below as a function of the reactor volume. The reactor
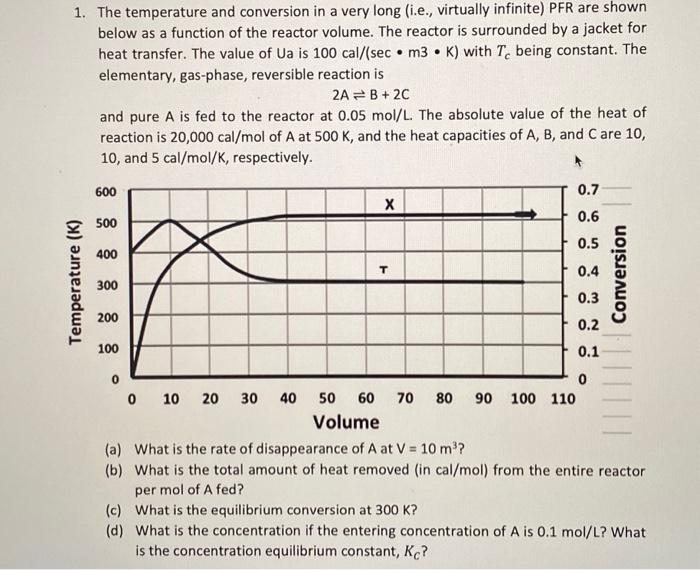
1. The temperature and conversion in a very long (i.e., virtually infinite) PFR are shown below as a function of the reactor volume. The reactor is surrounded by a jacket for heat transfer. The value of Ua is 100cal/(secm3K) with Tc being constant. The elementary, gas-phase, reversible reaction is 2AB+2C and pure A is fed to the reactor at 0.05mol/L. The absolute value of the heat of reaction is 20,000cal/mol of A at 500K, and the heat capacities of A,B, and C are 10 , 10 , and 5cal/mol/K, respectively. (a) What is the rate of disappearance of A at V=10m3 ? (b) What is the total amount of heat removed (in cal/mol) from the entire reactor per mol of A fed? (c) What is the equilibrium conversion at 300K ? (d) What is the concentration if the entering concentration of A is 0.1mol/L ? What is the concentration equilibrium constant, KC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


