Question: 1. Write down the Eutectoid reaction for Fe-Fe3C phase diagram (state the reaction, temperature and composition). 2. Write down the solubility limit of carbon (C)
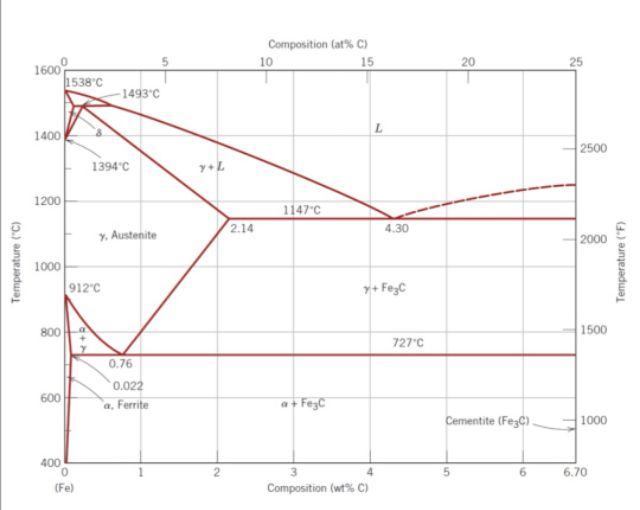
1. Write down the Eutectoid reaction for Fe-Fe3C phase diagram (state the reaction, temperature and composition). 2. Write down the solubility limit of carbon (C) in ferrite (-Fe) and austenite (). 3. Describe the role of carbon in iron (Fe) atomic structure for steel. 4. Compare the strength of steels containing 0.6wt%C and 1.2wt% C. Justify your answer. 5. Describe austenitizing temperature in heat treatment.
6. If we heat two steels with composition of 0.3wt% C at 760oC and the other one at 800oC, then quenched both steels in water to room temperature, which one will have better strength? Justify your answer. 7. Make a full phase analysis for a steel with composition of 1.2wt% C at these following temperatures i. 800oC ii. 727oC + T iii. 727oC T
Composition lat%C) 10 15 20 25 1600 1538C 1493C 1400 2500 1394"C y. 1200 1147C 2.14 4.30 y Austenite 2000 Temperature (C) 1000 912"c Temperature (F) + Fezc 800 1500 727C 0.76 0.022 a Ferrite 600 a + Fego Cementite (Fez) 1000 400 0 (Fe) 6.70 Composition (wt%C)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


