Question: 1. Write the Keq expression for the equilibrium reaction shown below: HF(aq)+H2O(I)H3O+(aq)+F(aq) 2. Given that Keq for the reaction shown below is 5.0109 at 25C,
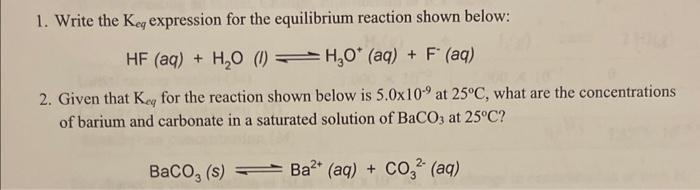
1. Write the Keq expression for the equilibrium reaction shown below: HF(aq)+H2O(I)H3O+(aq)+F(aq) 2. Given that Keq for the reaction shown below is 5.0109 at 25C, what are the concentrations of barium and carbonate in a saturated solution of BaCO3 at 25C ? BaCO3(s)Ba2+(aq)+CO32(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


