Question: 14. Write ionization equations and base ionization constant expressions for the following bases. a. hexylamine (C6H0NH2) c. carbonate ion (CO32) b. propylamine (C3H2NH2) d. hydrogen
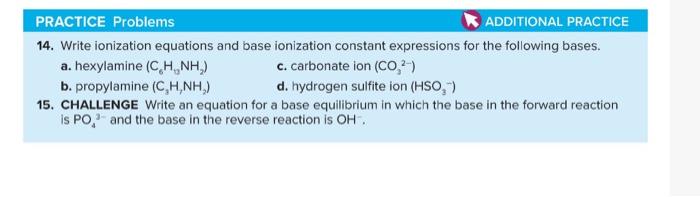
14. Write ionization equations and base ionization constant expressions for the following bases. a. hexylamine (C6H0NH2) c. carbonate ion (CO32) b. propylamine (C3H2NH2) d. hydrogen sulfite ion (HSO3) 15. CHALLENGE Write an equation for a base equilibrium in which the base in the forward reaction is PO43 and the base in the reverse reaction is OH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


