Question: 1a. Assuming the experiment was executed without error, 1b. Use the starting arnount of Mg,0.390g. and the result from Getermine the mass of MgO produced
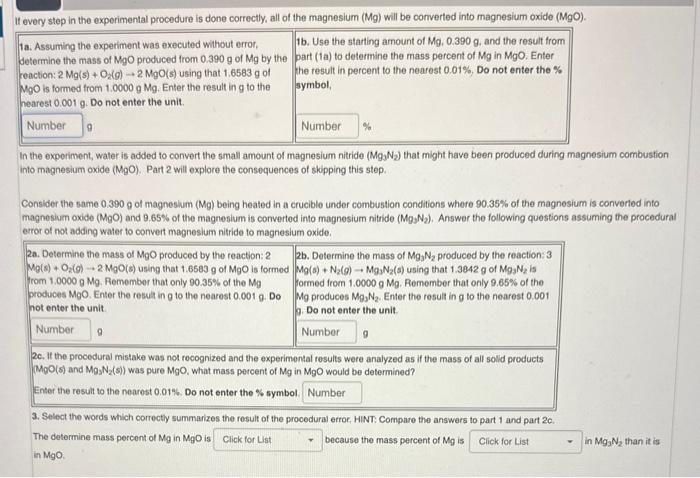
1a. Assuming the experiment was executed without error, 1b. Use the starting arnount of Mg,0.390g. and the result from Getermine the mass of MgO produced from 0.390g of Mg by the part (1a) to determine the mass percent of Mg in MgO. Enter reaction: 2Mg(s)+O2(g)2MgO(s) using that 1.6583g of MgO is formed trom 1.0000gMg. Enter the result in g to the the result in percent to the nearest 0.01%, Do not enter the % hearest 0.001g. Do not enter the unit. In the experiment, water is added to convert the small amount of magnesium nitride (Mg3N2) that might have been produced during magnesium combustion into magnesium oxide ( MgO). Part 2 will explore the consequences of skipping this step. Consiber the same 0.390g of magneslum (Mg) being heated in a crucible under combustion conditions where 90.35% of the magnesium is converted into magnesium oxide (MgO) and 9.65% of the magnesium is converted into magnesium nittide (Mgg2N2). Answer the following questions assuming the procedural error of not adding water to convert magneslum nitride to magnesium oxide. 2c. It the procedural mistake was not recognized and the experimental results were analyzed as if the mass of all solld products (MgO(s) and MgSN2(s) ) was pure MgO, what mass percent of Mg in MgO would be determined? Enter the result to the nearost 0.01%, Do not enter the % symbol. 3. Select the words which correctly summarizes the result of the procedural error. HINT: Compare the answers to part 1 and part 20. The determine mass percent of Mg in MgO is because the mass percent of Mg is in MgO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


