Question: 1a. Assuming the experiment was executed without error, determine the mass of MgO produced from 0.388g of Mg by the 1b. Use the starting amount
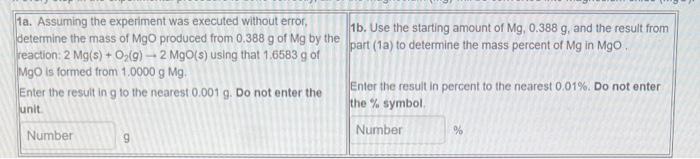
1a. Assuming the experiment was executed without error, determine the mass of MgO produced from 0.388g of Mg by the 1b. Use the starting amount of Mg,0.388g, and the result from reaction: 2Mg(s)+O2(g)2MgO(s) using that 1.6583g of part (1a) to determine the mass percent of Mg in MgO. MgO is formed from 1.0000gMg. Enter the result in g to the nearest 0.001g. Do not enter the Enter the result in percent to the nearest 0.01%. Do not enter unit. the % symbol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


