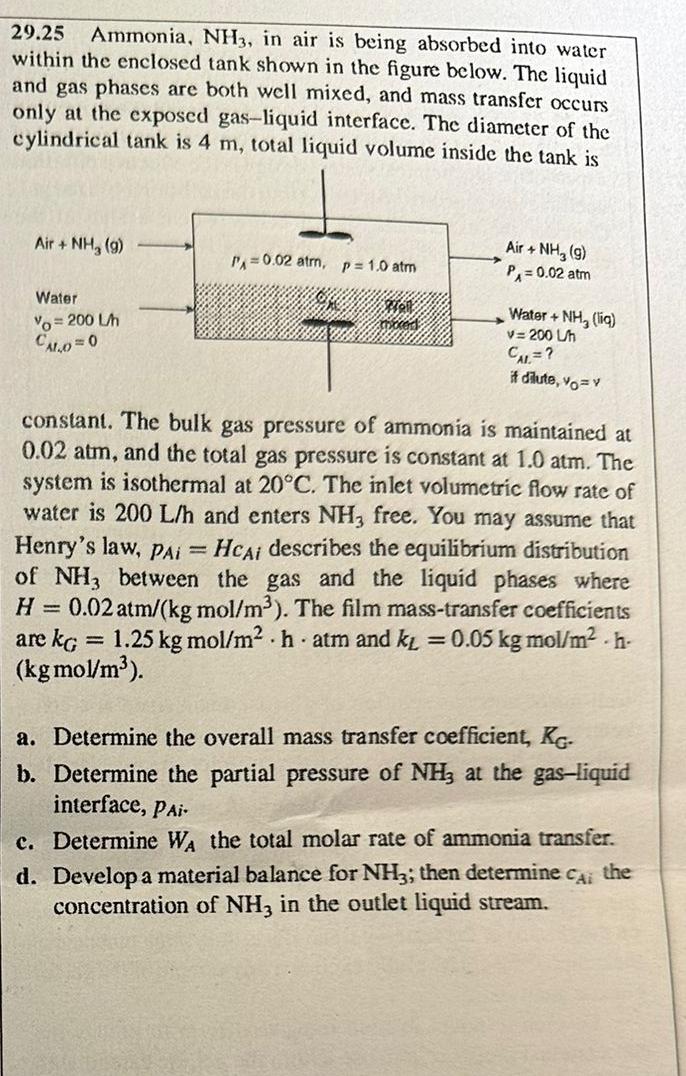
Question: 2 9 . 2 5 Ammonia, N H 3 , in air is being absorbed into water within the enclosed tank shown in the figure
Ammonia, in air is being absorbed into water within the enclosed tank shown in the figure below. The liquid and gas phases are both well mixed, and mass transfer occurs only at the exposed gasliquid interface. The diameter of the cylindrical tank is total liquid volume inside the tank is
constant. The bulk gas pressure of ammonia is maintained at atm, and the total gas pressure is constant at atm. The system is isothermal at The inlet volumetric flow rate of water is and enters free. You may assume that Henry's law, describes the equilibrium distribution of between the gas and the liquid phases where The film masstransfer coefficients are kgmo atm and kgmo
a Determine the overall mass transfer coefficient,
b Determine the partial pressure of at the gasliquid interface,
c Determine the total molar rate of ammonia transfer.
d Develop a material balance for ; then determine the concentration of in the outlet liquid stream.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


