Question: 2. A second order, irreversible reaction A+BC+D is to be carried out in an isothermal, partially mixed reactor. Tracer experiments show that the RTD for
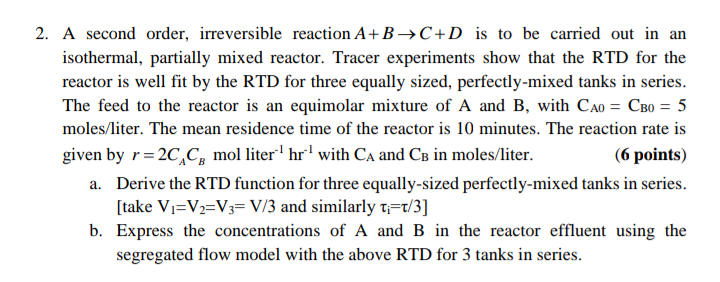
2. A second order, irreversible reaction A+BC+D is to be carried out in an isothermal, partially mixed reactor. Tracer experiments show that the RTD for the reactor is well fit by the RTD for three equally sized, perfectly-mixed tanks in series. The feed to the reactor is an equimolar mixture of A and B, with CA0=CB0=5 moles/liter. The mean residence time of the reactor is 10 minutes. The reaction rate is given by r=2CACB mol liter 1hr1 with CA and CB in moles/liter. (6 points) a. Derive the RTD function for three equally-sized perfectly-mixed tanks in series. [take V1=V2=V3=V/3 and similarly i=/3 ] b. Express the concentrations of A and B in the reactor effluent using the segregated flow model with the above RTD for 3 tanks in series
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


