Question: ( 2 ) Benzene and toluene form an ideal solution at 2 0 C , but a nonideal solution at 1 2 0 C .
Benzene and toluene form an ideal solution at but a nonideal solution at For the latter
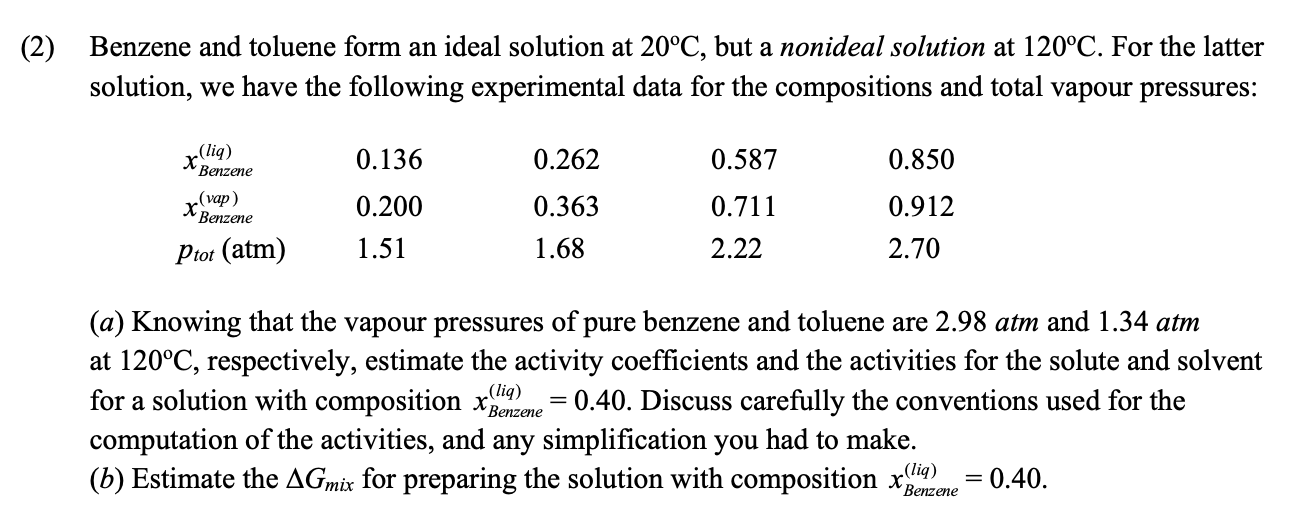
solution, we have the following experimental data for the compositions and total vapour pressures:
a Knowing that the vapour pressures of pure benzene and toluene are atm and atm
at respectively, estimate the activity coefficients and the activities for the solute and solvent
for a solution with composition Discuss carefully the conventions used for the
computation of the activities, and any simplification you had to make.
b Estimate the for preparing the solution with composition
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


