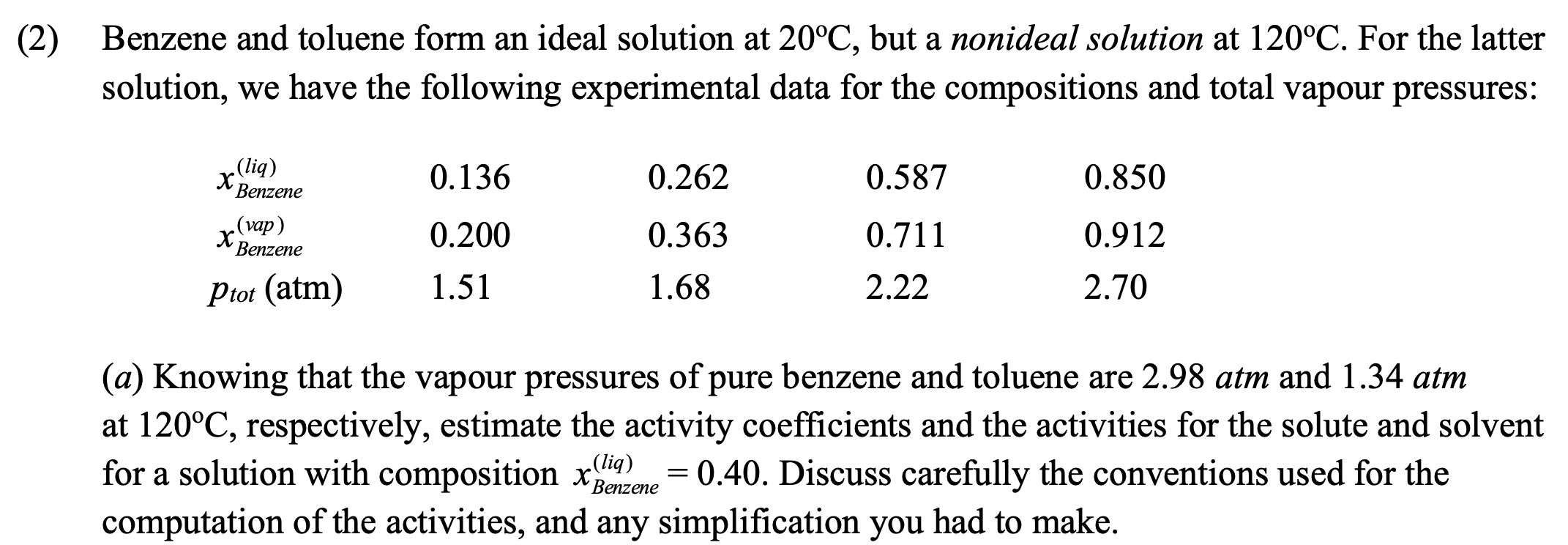
Question: ( 2 ) Benzene and toluene form an ideal solution at 2 0 C , but a nonideal solution at 1 2 0 C .
Benzene and toluene form an ideal solution at but a nonideal solution at For the latter
solution, we have the following experimental data for the compositions and total vapour pressures:
a Knowing that the vapour pressures of pure benzene and toluene are atm and atm
at respectively, estimate the activity coefficients and the activities for the solute and solvent
for a solution with composition Discuss carefully the conventions used for the
computation of the activities, and any simplification you had to make.
Can you make the plot on excel and solve by interpolation for mole fraction of toluene by using the mole fraction of benzene X
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


