Question: 2. Consider the following reaction system A B (1) B+ M C (2) M is a species present in much higher concentrations than A or
2. Consider the following reaction system A B (1) B+ M C (2) M is a species present in much higher concentrations than A or B, so in essence [M] ~ constant. The concentrations of B and C are 0 at t=0. Plot the concentrations of A, B and C as a function of time (in minutes) for the following sets of conditions:
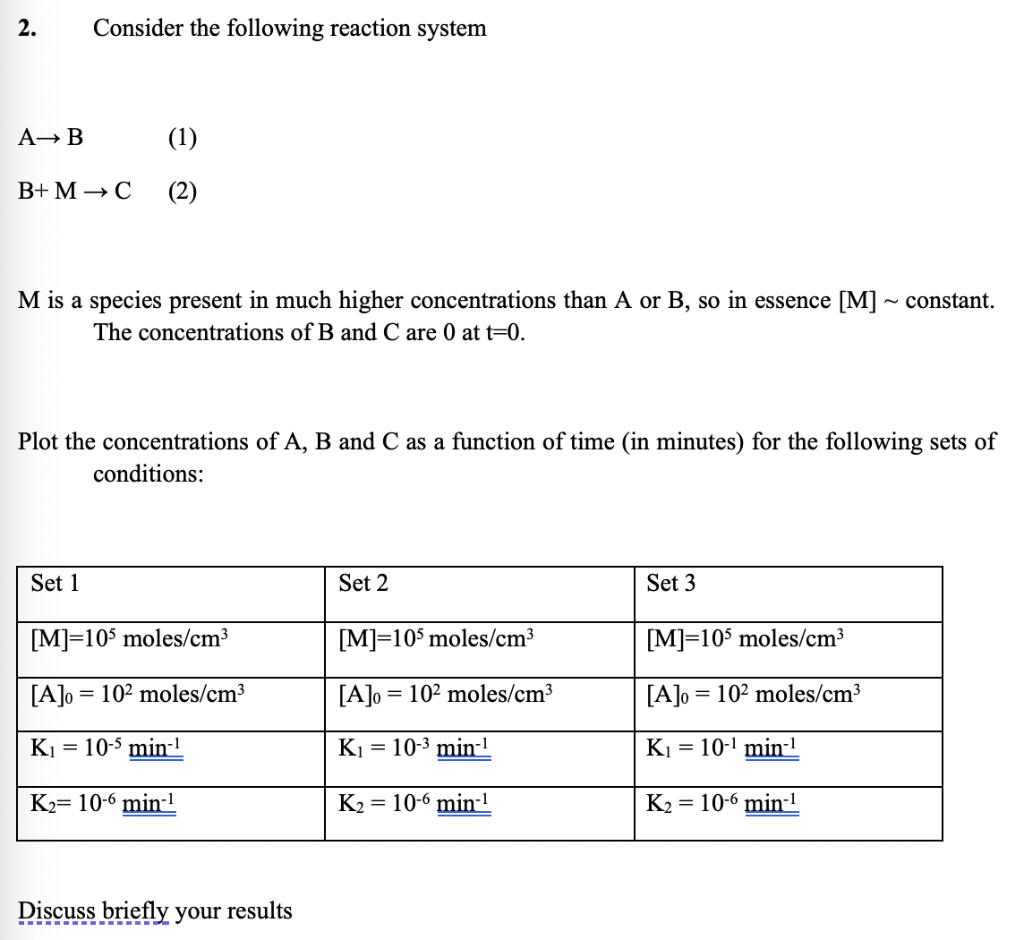
2. Consider the following reaction system A+B (1) B+ MC (2) M is a species present in much higher concentrations than A or B, so in essence [M] ~ constant. The concentrations of B and Care 0 at t=0. Plot the concentrations of A, B and C as a function of time (in minutes) for the following sets of conditions: Set 1 Set 2 Set 3 [M]=105 moles/cm3 [M]=105 moles/cm3 [M]=105 moles/cm3 [A]o = 102 moles/cm3 [A]o = 102 moles/cm3 [A]o = 102 moles/cm3 Ki = 10-5 min-1 Ki = 10-3 min-1 Ki = 10-1 min-1 K2= 10-6 min-1 K2 = 10-6 min-1 K2 = 10-6 min-1 Discuss briefly your results
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


