Question: 2. The excess Gibbs energy for a binary mixture is given by Gex = x1x2(a + bT), where a and b are constants and
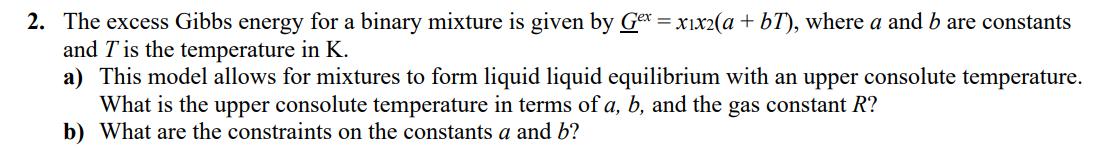
2. The excess Gibbs energy for a binary mixture is given by Gex = x1x2(a + bT), where a and b are constants and 7 is the temperature in K. a) This model allows for mixtures to form liquid liquid equilibrium with an upper consolute temperature. What is the upper consolute temperature in terms of a, b, and the gas constant R? b) What are the constraints on the constants a and b?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


