Question: 2) The unit cell for an ionic solid is shown below. The cation forms the lattice and the anion occupies the voids. Answer the following
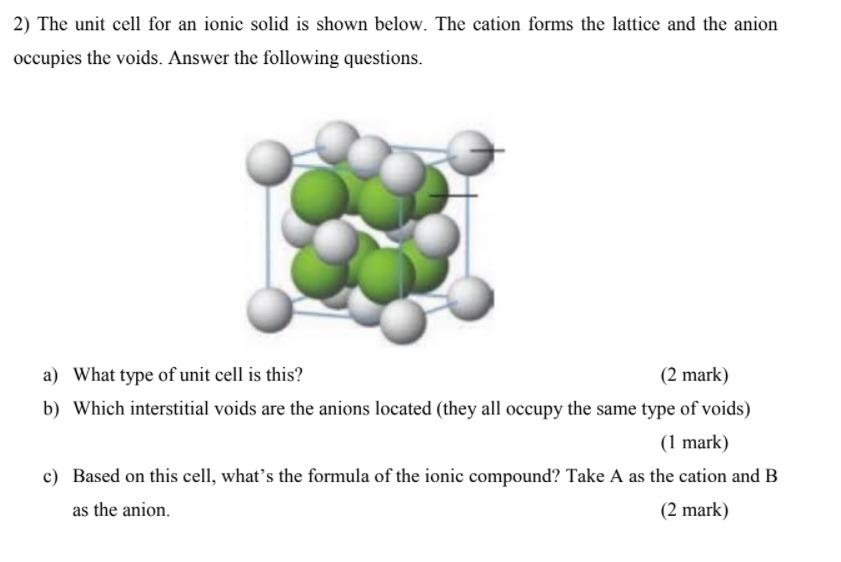
2) The unit cell for an ionic solid is shown below. The cation forms the lattice and the anion occupies the voids. Answer the following questions. a) What type of unit cell is this? (2 mark) b) Which interstitial voids are the anions located (they all occupy the same type of voids) (1 mark) c) Based on this cell, what's the formula of the ionic compound? Take A as the cation and B as the anion. (2 mark)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


